Cancer Res Treat.
2019 Jan;51(1):194-210. 10.4143/crt.2018.031.
Cancer-Associated Fibroblasts Promote the Chemo-resistance in Gastric Cancer through Secreting IL-11 Targeting JAK/STAT3/Bcl2 Pathway
- Affiliations
-
- 1Department of Gastrointestinal and Pancreatic Surgery, Zhejiang Provincial People's Hospital, People's Hospital of Hangzhou Medical College, Hangzhou, China. yp66self@163.com
- 2Key Laboratory of Gastroenterology of Zhejiang Province, Hangzhou, China.
- 3Department of General Surgery, Ningbo No. 2 Hospital, Ningbo, China.
- KMID: 2437613
- DOI: http://doi.org/10.4143/crt.2018.031
Abstract
- PURPOSE
Our aim was to detect the potential role of interleukin 11 (IL-11) in the development of chemo-resistance in gastric cancer and to reveal the mechanism involved in the process.
MATERIALS AND METHODS
Here, we used flow cytometry to examine the percentage of cancer-associated-fibroblasts in tumor samples from chemo-resistant and -sensitive gastric cancer patients. Using MTT assay, we detected the cell viability under different conditions. Using quantitative real-time polymerase chain reaction and Western blotting, we determined the target expressions in mRNA and protein levels. We also performed immunohistochemistry and immunofluorescence to detect the target proteins under different conditions. Animal models were constructed to verify the potential role of IL-11 in chemo-resistant develop in vivo.
RESULTS
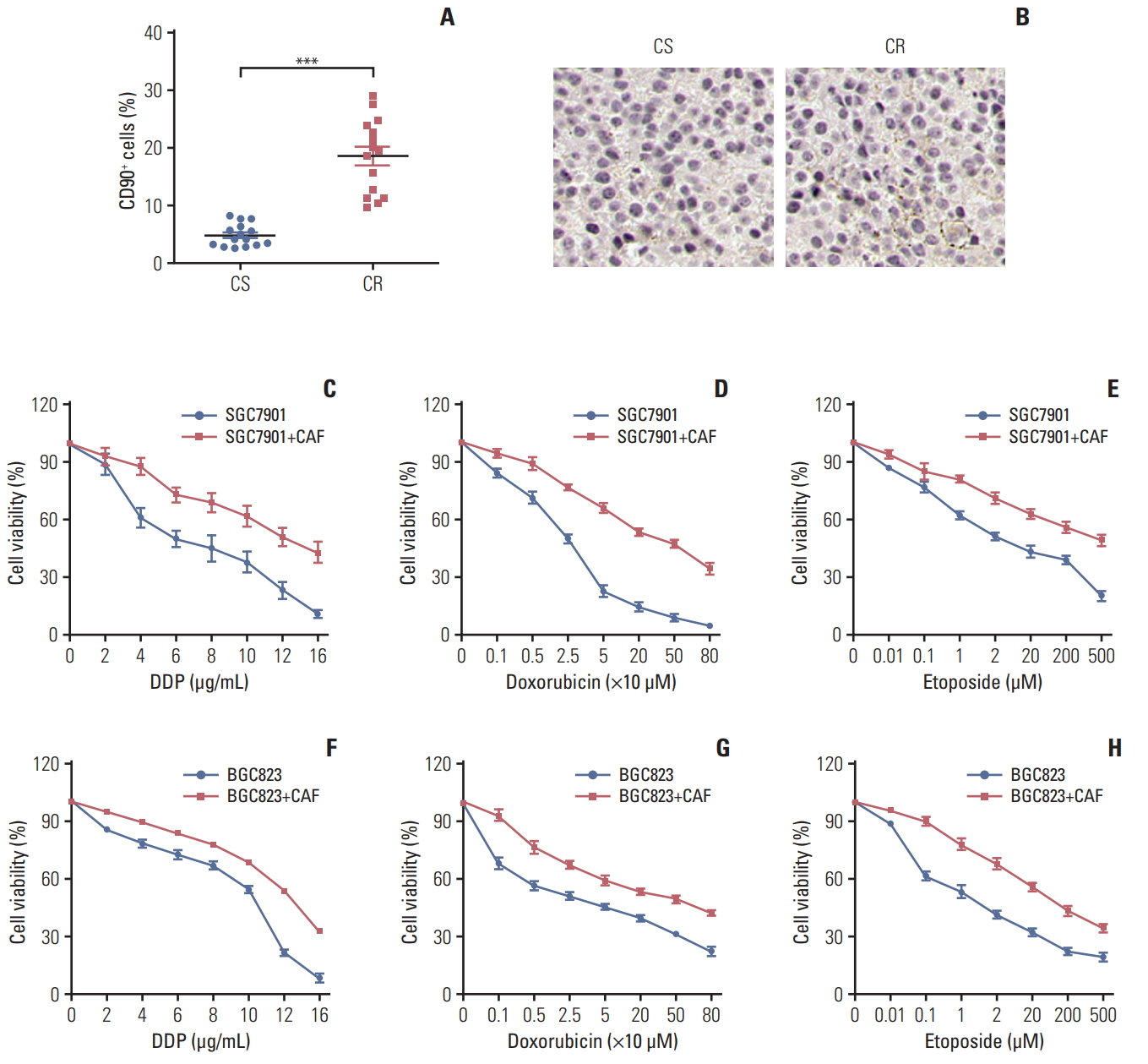
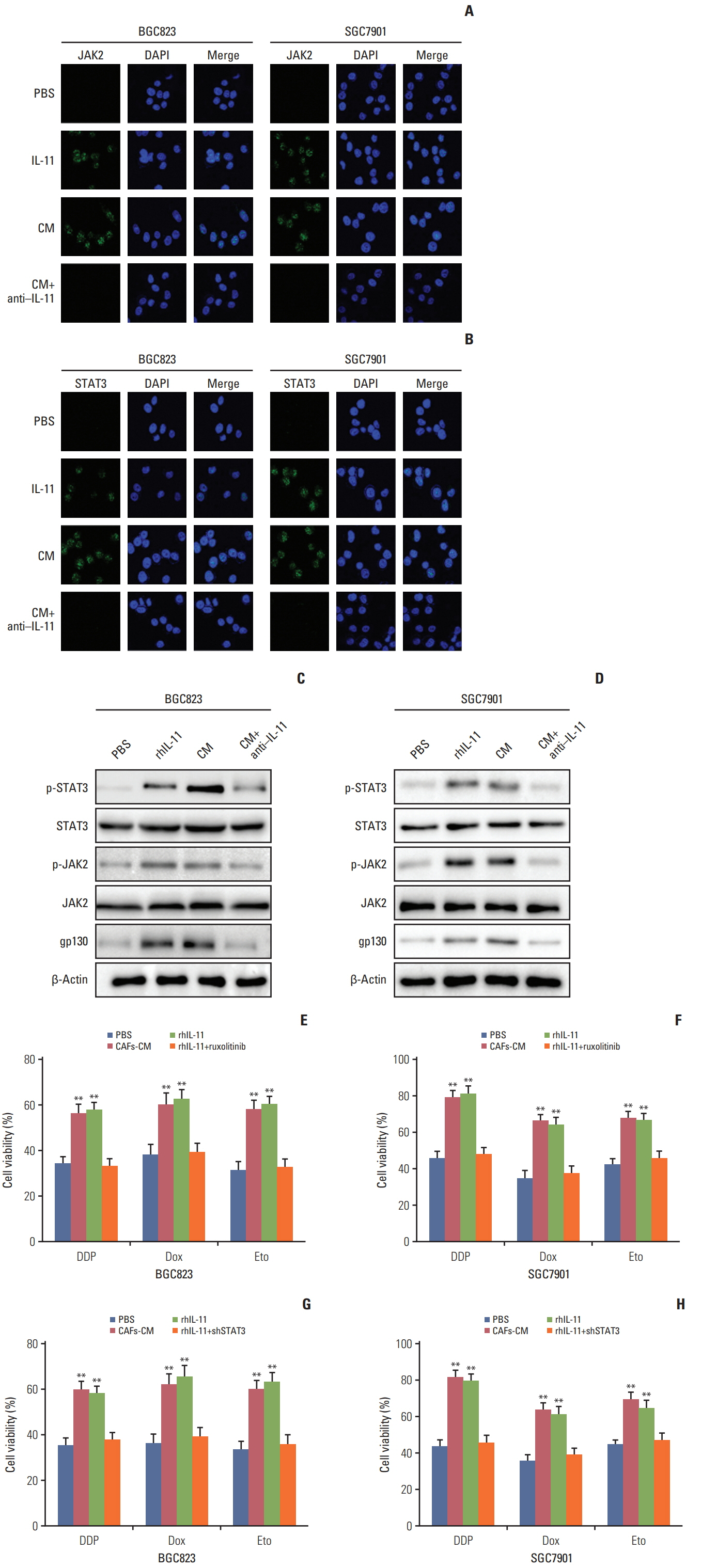
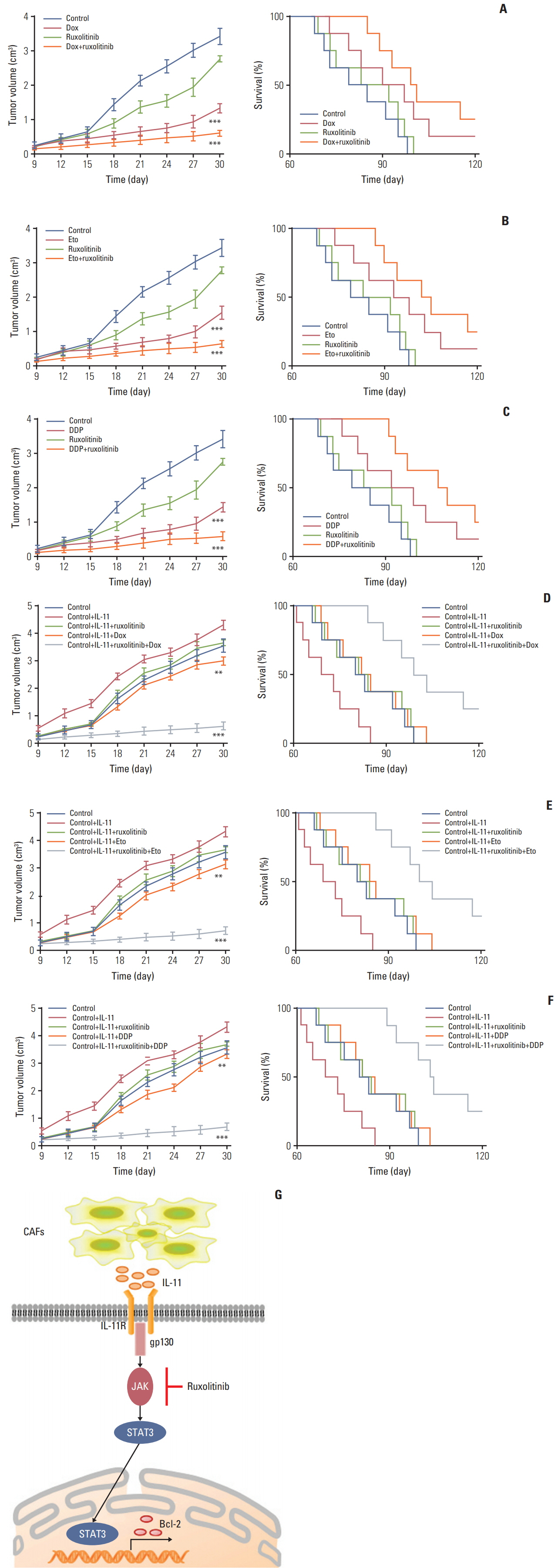
Herein, we observed enriched cancer associated fibroblasts in drug resistant tumor tissues from gastric patients. Those fibroblasts facilitate the chemotherapeutic drugs resistance development through the secretion of IL-11, which activates the IL-11/IL-11R/gp130/JAK/STAT3 anti-apoptosis signaling pathway in gastric cancer cells. We found that the combination of chemotherapeutic drugs and JAK inhibitor overcomes the resistance and increases the survival of mice with gastric cancer xenografts.
CONCLUSION
Ourresults demonstrated that IL-11 contributed to the obtain ofresistance to chemotherapy drugs through gp130/JAK/STAT3/Bcl2 pathway, and targeting the IL-11 signaling pathway induced by fibroblasts might be a promising strategy to overcome the multi-drugs resistant cancer in clinic.
MeSH Terms
Figure
Reference
-
References
1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66:115–32.
Article2. Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013; 13:714–26.
Article3. Juan TY, Roffler SR, Hou HS, Huang SM, Chen KC, Leu YL, et al. Antiangiogenesis targeting tumor microenvironment synergizes glucuronide prodrug antitumor activity. Clin Cancer Res. 2009; 15:4600–11.
Article4. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013; 19:1423–37.
Article5. Kikuchi K, McNamara KM, Miki Y, Moon JY, Choi MH, Omata F, et al. Effects of cytokines derived from cancer-associated fibroblasts on androgen synthetic enzymes in estrogen receptor-negative breast carcinoma. Breast Cancer Res Treat. 2017; 166:709–23.
Article6. Chua AC, Klopcic BR, Ho DS, Fu SK, Forrest CH, Croft KD, et al. Dietary iron enhances colonic inflammation and IL-6/IL-11-Stat3 signaling promoting colonic tumor development in mice. PLoS One. 2013; 8:e78850.
Article7. Putoczki TL, Ernst M. IL-11 signaling as a therapeutic target for cancer. Immunotherapy. 2015; 7:441–53.
Article8. Tao L, Huang G, Wang R, Pan Y, He Z, Chu X, et al. Cancer-associated fibroblasts treated with cisplatin facilitates chemoresistance of lung adenocarcinoma through IL-11/IL-11R/STAT3 signaling pathway. Sci Rep. 2016; 6:38408.
Article9. Johnstone CN, Chand A, Putoczki TL, Ernst M. Emerging roles for IL-11 signaling in cancer development and progression: focus on breast cancer. Cytokine Growth Factor Rev. 2015; 26:489–98.
Article10. Sanchez-Lopez E, Flashner-Abramson E, Shalapour S, Zhong Z, Taniguchi K, Levitzki A, et al. Targeting colorectal cancer via its microenvironment by inhibiting IGF-1 receptor-insulin receptor substrate and STAT3 signaling. Oncogene. 2016; 35:2634–44.
Article11. Zheng H, Yang Y, Han J, Jiang WH, Chen C, Wang MC, et al. TMED3 promotes hepatocellular carcinoma progression via IL-11/STAT3 signaling. Sci Rep. 2016; 6:37070.
Article12. Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008; 68:918–26.
Article13. Johnson P, Beswick EJ, Chao C, Powell DW, Hellmich MR, Pinchuk IV. Isolation of CD 90+ fibroblast/myofibroblasts from human frozen gastrointestinal specimens. J Vis Exp. 2016; e53691.
Article14. Johnston A, Xing X, Guzman AM, Riblett M, Loyd CM, Ward NL, et al. IL-1F5, -F6, -F8, and -F9: a novel IL-1 family signaling system that is active in psoriasis and promotes keratinocyte antimicrobial peptide expression. J Immunol. 2011; 186:2613–22.
Article15. Varas-Godoy M, Rice G, Illanes SE. The crosstalk between ovarian cancer stem cell niche and the tumor microenvironment. Stem Cells Int. 2017; 2017:5263974.
Article16. Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010; 148:135–46.
Article17. Paraiso KH, Smalley KS. Fibroblast-mediated drug resistance in cancer. Biochem Pharmacol. 2013; 85:1033–41.
Article18. Flach EH, Rebecca VW, Herlyn M, Smalley KS, Anderson AR. Fibroblasts contribute to melanoma tumor growth and drug resistance. Mol Pharm. 2011; 8:2039–49.
Article19. Cavaco A, Rezaei M, Niland S, Eble JA. Collateral damage intended-cancer-associated fibroblasts and vasculature are potential targets in cancer therapy. Int J Mol Sci. 2017; 18:E2355.
Article20. Gal P, Varinska L, Faber L, Novak S, Szabo P, Mitrengova P, et al. How signaling molecules regulate tumor microenvironment: parallels to wound repair. Molecules. 2017; 22:E1818.
Article21. Setrerrahmane S, Xu H. Tumor-related interleukins: old validated targets for new anti-cancer drug development. Mol Cancer. 2017; 16:153.
Article22. Ren C, Chen Y, Han C, Fu D, Chen H. Plasma interleukin-11 (IL-11) levels have diagnostic and prognostic roles in patients with pancreatic cancer. Tumour Biol. 2014; 35:11467–72.
Article23. De Araujo RF Jr, Pessoa JB, Cruz LJ, Chan AB, De Castro Miguel E, Cavalcante RS, et al. Apoptosis in human liver carcinoma caused by gold nanoparticles in combination with carvedilol is mediated via modulation of MAPK/Akt/mTOR pathway and EGFR/FAAD proteins. Int J Oncol. 2018; 52:189–200.
Article24. Guerriero L, Palmieri G, De Marco M, Cossu A, Remondelli P, Capunzo M, et al. The anti-apoptotic BAG3 protein is involved in BRAF inhibitor resistance in melanoma cells. Oncotarget. 2017; 8:80393–404.
Article25. Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010; 10:116–29.
Article26. Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med. 2012; 18:1359–68.
Article27. Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012; 487:500–4.
Article28. Baudino TA. Targeted cancer therapy: the next generation of cancer treatment. Curr Drug Discov Technol. 2015; 12:3–20.
Article29. Coley HM. Overcoming multidrug resistance in cancer: clinical studies of p-glycoprotein inhibitors. Methods Mol Biol. 2010; 596:341–58.
Article30. Zhu W, Li Y, Liu L, Chen Y, Xi F. Supramolecular hydrogels as a universal scaffold for stepwise delivering Dox and Dox/cisplatin loaded block copolymer micelles. Int J Pharm. 2012; 437:11–9.
Article31. Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009; 9:665–74.
Article32. Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ, Feng YM. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-beta signalling. Br J Cancer. 2014; 110:724–32.33. Nakayama T, Yoshizaki A, Izumida S, Suehiro T, Miura S, Uemura T, et al. Expression of interleukin-11 (IL-11) and IL-11 receptor alpha in human gastric carcinoma and IL-11 upregulates the invasive activity of human gastric carcinoma cells. Int J Oncol. 2007; 30:825–33.34. Ernst M, Najdovska M, Grail D, Lundgren-May T, Buchert M, Tye H, et al. STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest. 2008; 118:1727–38.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glutamine Deprivation Causes Hydrogen Peroxide-induced Interleukin-8 Expression via Jak1/Stat3 Activation in Gastric Epithelial AGS Cells
- Role of Janus Kinase/Signal Transducers and Activators of Transcription in the Pathogenesis of Pancreatitis and Pancreatic Cancer
- Janus Kinase 2 Inhibitor AG490 Inhibits the STAT3 Signaling Pathway by Suppressing Protein Translation of gp130
- NDRG2-mediated Modulation of SOCS3 and STAT3 Activity Inhibits IL-10 Production
- Reversal of Cisplatin Resistance by Epigallocatechin Gallate Is Mediated by Downregulation of Axl and Tyro 3 Expression in Human Lung Cancer Cells