Neonatal Med.
2018 Nov;25(4):153-160. 10.5385/nm.2018.25.4.153.
Tolerability and Effect of Early High-Dose Amino Acid Administration in Extremely Low Birth Weight Infants
- Affiliations
-
- 1Department of Pediatrics, Korea University College of Medicine, Seoul, Korea.
- 2Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. sein.sung@samsung.com
- KMID: 2436133
- DOI: http://doi.org/10.5385/nm.2018.25.4.153
Abstract
- PURPOSE
The aim of this study is to examine the tolerability and effect of early highdose amino acid administration in extremely low birth weight infants (ELBWIs).
METHODS
This retrospective cohort study included ELBWI (birth weight < 1,000 g, n=142). Biochemical, nutritional, and neurodevelopmental data were compared between infants who received conventional low amino acid (LAA; 1.5 g/kg/day) and those who received high amino acid (HAA; 3 g/kg/day) within the first 48 hours after birth. Neurodevelopmental data included weight, height, and head circumference at discharge, 12 to 14 and 18 to 24 months of corrected age and the Korean Bayley Scale of Infant Development II (K-BSID-II) score at 18 to 24 months of corrected age.
RESULTS
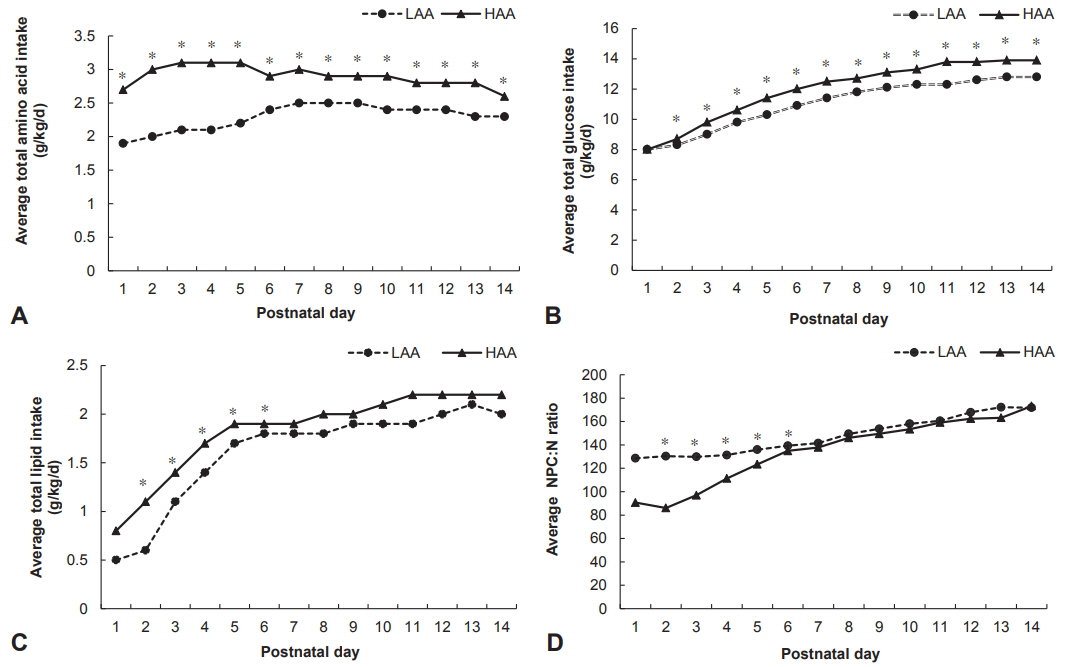
The HAA group demonstrated higher peak plasma albumin (3.0±0.4 vs. 3.2±0.5, P < 0.05) and lower serum creatinine (1.7±0.9 vs. 1.4±0.8, P < 0.05) during the first 14 days than the LAA group. Full enteral feeding was achieved significantly earlier in infants in the HAA group than in infants in the LAA group (46.2±23.0 days vs. 34.3±21 days, P < 0.01). There was no difference between the two groups in the z score changes in all growth indicators from birth to discharge and at 12 to 14 and 18 to 24 months of corrected age, as well as in the K-BSID-II score at 18 to 24 months of corrected age.
CONCLUSION
Aggressive administration of amino acids during the first 2 days of life in ELBWI was well tolerated and correlated with earlier full enteral feeding, but did not improve growth and neurodevelopment.
MeSH Terms
Figure
Reference
-
1. Ziegler EE, O'Donnell AM, Nelson SE, Fomon SJ. Body composition of the reference fetus. Growth. 1976; 40:329–41.2. Yang S, Lee BS, Park HW, Choi YS, Chung SH, Kim JH, et al. Effect of high vs standard early parenteral amino acid supplementation on the growth outcomes in very low birth weight infants. JPEN J Parenter Enteral Nutr. 2013; 37:327–34.3. Ziegler EE. Protein requirements of very low birth weight infants. J Pediatr Gastroenterol Nutr. 2007; 45 Suppl 3:S170–4.4. Brownlee KG, Kelly EJ, Ng PC, Kendall-Smith SC, Dear PR. Early or late parenteral nutrition for the sick preterm infant? Arch Dis Child. 1993; 69:281–3.5. Ho MY, Yen Yu, Hsieh MC, Chen HY, Chien SC, Hus-Lee SM. Early versus late nutrition support in premature neonates with respiratory distress syndrome. Nutrition. 2003; 19:257–60.6. Saini J, MacMahon P, Morgan JB, Kovar IZ. Early parenteral feeding of amino acids. Arch Dis Child. 1989; 64:1362–6.7. Shulman DI, Kanarek K. Gastrin, motilin, insulin, and insulin-like growth factor-I concentrations in very-low-birth-weight infants receiving enteral or parenteral nutrition. JPEN J Parenter Enteral Nutr. 1993; 17:130–3.8. Van Goudoever JB, Colen T, Wattimena JL, Huijmans JG, Carnielli VP, Sauer PJ. Immediate commencement of amino acid supplementation in preterm infants: effect on serum amino acid concentrations and protein kinetics on the first day of life. J Pediatr. 1995; 127:458–65.9. Hay WW Jr, Lucas A, Heird WC, Ziegler E, Levin E, Grave GD, et al. Workshop summary: nutrition of the extremely low birth weight infant. Pediatrics. 1999; 104:1360–8.10. Poindexter BB, Ehrenkranz RA, Stoll BJ, Koch MA, Wright LL, Oh W, et al. Effect of parenteral glutamine supplementation on plasma amino acid concentrations in extremely low-birthweight infants. Am J Clin Nutr. 2003; 77:737–43.11. Valentine CJ, Fernandez S, Rogers LK, Gulati P, Hayes J, Lore P, et al. Early amino-acid administration improves preterm infant weight. J Perinatol. 2009; 29:428–32.12. Ibrahim HM, Jeroudi MA, Baier RJ, Dhanireddy R, Krouskop RW. Aggressive early total parental nutrition in low-birth-weight infants. J Perinatol. 2004; 24:482–6.13. Thureen PJ, Melara D, Fennessey PV, Hay WW Jr. Effect of low versus high intravenous amino acid intake on very low birth weight infants in the early neonatal period. Pediatr Res. 2003; 53:24–32.14. Blanco CL, Gong AK, Green BK, Falck A, Schoolfield J, Liechty EA. Early changes in plasma amino acid concentrations during aggressive nutritional therapy in extremely low birth weight infants. J Pediatr. 2011; 158:543–8.15. Bulbul A, Okan F, Bulbul L, Nuhoglu A. Effect of low versus high early parenteral nutrition on plasma amino acid profiles in very low birth-weight infants. J Matern Fetal Neonatal Med. 2012; 25:770–6.16. Clark RH, Chace DH, Spitzer AR; Pediatrix Amino Acid Study Group. Effects of two different doses of amino acid supplementation on growth and blood amino acid levels in premature neonates admitted to the neonatal intensive care unit: a randomized, controlled trial. Pediatrics. 2007; 120:1286–96.17. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001; 163:1723–9.18. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013; 13:59.19. Hay WW, Thureen P. Protein for preterm infants: how much is needed? How much is enough? How much is too much? Pediatr Neonatol. 2010; 51:198–207.20. Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006; 117:1253–61.21. Franz AR, Pohlandt F, Bode H, Mihatsch WA, Sander S, Kron M, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics. 2009; 123:e101–9.22. Cooke RW. Are there critical periods for brain growth in children born preterm? Arch Dis Child Fetal Neonatal Ed. 2006; 91:F17–20.23. Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics. 2001; 107:E1.24. Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics. 2001; 107:270–3.25. Cooke RJ, Ainsworth SB, Fenton AC. Postnatal growth retardation: a universal problem in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2004; 89:F428–30.26. Sakurai M, Itabashi K, Sato Y, Hibino S, Mizuno K. Extrauterine growth restriction in preterm infants of gestational age < or =32 weeks. Pediatr Int. 2008; 50:70–5.27. Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics. 2003; 111(5 Pt 1):986–90.28. De Curtis M, Rigo J. Extrauterine growth restriction in very-low-birthweight infants. Acta Paediatr. 2004; 93:1563–8.29. Grantham-McGregor S. A review of studies of the effect of severe malnutrition on mental development. J Nutr. 1995; 125(8 Suppl):2233S–8S.30. Thureen PJ. Early aggressive nutrition in very preterm infants. Nestle Nutr Workshop Ser Pediatr Program. 2007; 59:193–204.31. te Braake FW, van den Akker CqH, Wattimena DJ, Huijmans JG, van Goudoever JB. Amino acid administration to premature infants directly after birth. J Pediatr. 2005; 147:457–61.32. Denne SC. Regulation of proteolysis and optimal protein accretion in extremely premature newborns. Am J Clin Nutr. 2007; 85:621S–4S.33. Anderson TL, Muttart CR, Bieber MA, Nicholson JF, Heird WC. A controlled trial of glucose versus glucose and amino acids in premature infants. J Pediatr. 1979; 94:947–51.34. Zlotkin SH, Bryan MH, Anderson GH. Intravenous nitrogen and energy intakes required to duplicate in utero nitrogen accretion in prematurely born human infants. J Pediatr. 1981; 99:115–20.35. Ridout E, Melara D, Rottinghaus S, Thureen PJ. Blood urea nitrogen concentration as a marker of amino-acid intolerance in neonates with birthweight less than 1250 g. J Perinatol. 2005; 25:130–3.36. Jadhav P, Parimi PS, Kalhan SC. Parenteral amino acid and metabolic acidosis in premature infants. JPEN J Parenter Enteral Nutr. 2007; 31:278–83.37. Trivedi A, Sinn JK. Early versus late administration of amino acids in preterm infants receiving parenteral nutrition. Cochrane Database Syst Rev. 2013; (7):CD008771.38. Burgess L, Morgan C, Mayes K, Tan M. Plasma arginine levels and blood glucose control in very preterm infants receiving 2 different parenteral nutrition regimens. JPEN J Parenter Enteral Nutr. 2014; 38:243–53.39. Morgan C, McGowan P, Herwitker S, Hart AE, Turner MA. Postnatal head growth in preterm infants: a randomized controlled parenteral nutrition study. Pediatrics. 2014; 133:e120–8.40. Neu J, Hauser N, Douglas-Escobar M. Postnatal nutrition and adult health programming. Semin Fetal Neonatal Med. 2007; 12:78–86.41. Masclee AA, Gielkens HA, Lam WF, de Boer SY, Lamers CB. Effects of parenteral nutrients on gastrointestinal motility and secretion. Scand J Gastroenterol Suppl. 1996; 218:50–5.42. Nealon WH, Upp JR Jr, Alexander RW, Gomez G, Townsend CM Jr, Thompson JC. Intravenous amino acids stimulate human gallbladder emptying and hormone release. Am J Physiol. 1990; 259(2 Pt 1):G173–8.43. Zoli G, Ballinger A, Healy J, O'Donnell LJ, Clark M, Farthing MJ. Promotion of gallbladder emptying by intravenous aminoacids. Lancet. 1993; 341:1240–1.44. Niederau C, Sonnenberg A, Erckenbrecht J. Effects of intravenous infusion of amino acids, fat, or glucose on unstimulated pancreatic secretion in healthy humans. Dig Dis Sci. 1985; 30:445–55.45. Snape WJ Jr, Yoo S. Effect of amino acids on isolated colonic smooth muscle from the rabbit. J Pharmacol Exp Ther. 1985; 235:690–5.46. Roggero P, Gianni ML, Liotto N, Taroni F, Morniroli D, Mosca F. Small for gestational age preterm infants: nutritional strategies and quality of growth after discharge. J Matern Fetal Neonatal Med. 2011; 24 Suppl 1:144–6.47. Hack M, Schluchter M, Cartar L, Rahman M, Cuttler L, Borawski E. Growth of very low birth weight infants to age 20 years. Pediatrics. 2003; 112(1 Pt 1):e30–8.48. Cetin I, Corbetta C, Sereni LP, Marconi AM, Bozzetti P, Pardi G, et al. Umbilical amino acid concentrations in normal and growth-retarded fetuses sampled in utero by cordocentesis. Am J Obstet Gynecol. 1990; 162:253–61.49. Denne SC. Protein and energy requirements in preterm infants. Semin Neonatol. 2001; 6:377–82.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Blood Urea Nitrogen Concentration and Aggressive Parenteral Amino Acid Administration in Extremely Low Birth Weight Infants during the First Week
- Corrigendum: Tolerability and Effect of Early High-Dose Amino Acid Administration in Extremely Low Birth Weight Infants
- Nutritional strategy of early amino acid administration in very low birth weight infants
- Late and Insufficient Phosphorus Supplementation is Associated with Early Severe Hypophosphatemia in Extremely Low Birth Weight Infants with Early Amino Acid Administration
- Comparison of total parenteral nutrition-associated cholestasis according to amino acid mixtures in very low birth weight infants