Anesth Pain Med.
2018 Jul;13(3):248-255. 10.17085/apm.2018.13.3.248.
Anesthesia research in the artificial intelligence era
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea. spss@snuh.org
- KMID: 2436031
- DOI: http://doi.org/10.17085/apm.2018.13.3.248
Abstract
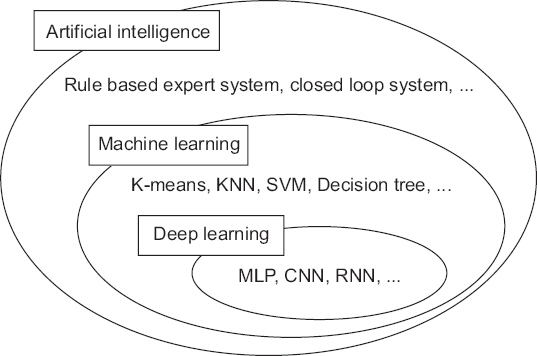
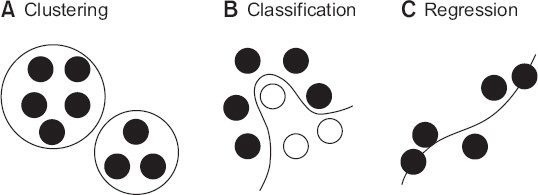
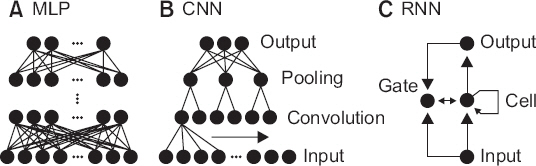
- A noteworthy change in recent medical research is the rapid increase of research using big data obtained from electrical medical records (EMR), order communication systems (OCS), and picture archiving and communication systems (PACS). It is often difficult to apply traditional statistical techniques to research using big data because of the vastness of the data and complexity of the relationships. Therefore, the application of artificial intelligence (AI) techniques which can handle such problems is becoming popular. Classical machine learning techniques, such as k-means clustering, support vector machine, and decision tree are still efficient and useful for some research problems. The deep learning techniques, such as multi-layer perceptron, convolutional neural network, and recurrent neural network have been spotlighted by the success of deep belief networks and convolutional neural networks in solving various problems that are difficult to solve by conventional methods. The results of recent research using artificial intelligence techniques are comparable to human experts. This article introduces technologies that help researchers conduct medical research and understand previous literature in the era of AI.
MeSH Terms
Figure
Reference
-
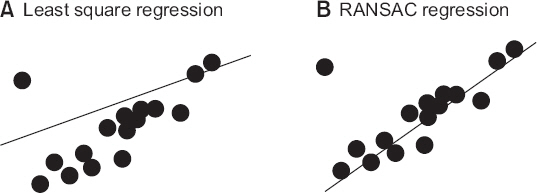
1. Ehteshami Bejnordi B, Veta M, Johannes van Diest P, van Ginneken B, Karssemeijer N, Litjens G, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017; 318:2199–210. DOI: 10.1001/jama.2017.14585. PMID: 29234806. PMCID: PMC5820737.2. Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016; 316:2402–10. DOI: 10.1001/jama.2016.17216. PMID: 27898976.3. Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017; 542:115–8. DOI: 10.1038/nature21056. PMID: 28117445.4. Bickford RG. Automatic electroencephalographic control of general anesthesia. Electroencephalogr Clin Neurophysiol. 1950; 2:93–6. DOI: 10.1016/0013-4694(50)90014-9.5. Pasin L, Nardelli P, Pintaudi M, Greco M, Zambon M, Cabrini L, et al. Closed-loop delivery systems versus manually controlled administration of total IV anesthesia. Anesth Analg. 2017; 124:456–64. DOI: 10.1213/ANE.0000000000001394. PMID: 28099320.6. Cotoia A, Mirabella L, Beck R, Matrella P, Assenzo V, Chazot T, et al. Effects of closed-loop intravenous anesthesia guided by bispectral index in adult patients on emergence delirium: a randomized controlled study. Minerva Anestesiol. 2018; 84:437–46. PMID: 29239148.7. Srinivasa S, Kahokehr A, Soop M, Taylor M, Hill AG. Goal-directed fluid therapy- a survey of anaesthetists in the UK, USA, Australia and New Zealand. BMC Anesthesiol. 2013; 13:5. DOI: 10.1186/1471-2253-13-5. PMID: 23433064. PMCID: PMC3598228.8. Joosten A, Coeckelenbergh S, Delaporte A, Ickx B, Closset J, Roumeguere T, et al. Implementation of closed-loop-assisted intra-operative goal-directed fluid therapy during major abdominal surgery: a case-control study with propensity matching. Eur J Anaesthesiol. 2018; doi:10.1097/EJA.0000000000000827. [Epub ahead of print]. DOI: 10.1097/EJA.0000000000000827.9. De Jonckheere J, Delecroix M, Jeanne M, Keribedj A, Couturier N, Logier R. Automated analgesic drugs delivery guided by vagal tone evaluation: interest of the analgesia nociception index (ANI). Conf Proc IEEE Eng Med Biol Soc. 2013; 2013:1952–5. DOI: 10.1109/EMBC.2013.6609910.10. Ngan Kee WD, Tam YH, Khaw KS, Ng FF, Lee SWY. Closed-loop feedback computer-controlled phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery: a randomized trial comparing automated boluses versus infusion. Anesth Analg. 2017; 125:117–23. DOI: 10.1213/ANE.0000000000001974. PMID: 28368936.11. Joosten A, Jame V, Alexander B, Chazot T, Liu N, Cannesson M, et al. Feasibility of fully automated hypnosis, analgesia, and fluid management using 2 independent closed-loop systems during major vascular surgery: a pilot study. Anesth Analg. 2018; doi:10.1213/ANE.0000000000003433. [Epub ahead of print]. DOI: 10.1213/ANE.0000000000003433.12. Goudra B, Singh PM. Failure of sedasys: destiny or poor design? Anesth Analg. 2017; 124:686–8. DOI: 10.1213/ANE.0000000000001643. PMID: 27861440.13. Bibian S. Closed loop total intravenous anesthesia (TIVA) for combat casualty care - an auto-pilot for safe and effective TIVA delivery. Paper presented at: Society of Technology in Anesthesia Annual Meeting 2018; 2018 Jan 10-13. Aventura (FL), USA: Available from http://www.stahq.org/userfiles/ckfiles/files/STA_18AM_Handout_Bibian.pdf.14. Reinsel D, Gantz J, Rydning J. Data age 2025: the evolution of data to life-critical. International Data Corporation [serial on the Internet]. 2017. [2018 Jun 5]. Available from https://www.seagate.com/www-content/our-story/trends/files/Seagate-WP-DataAge2025-March-2017.pdf.15. Sessler DI, Sigl JC, Manberg PJ, Kelley SD, Schubert A, Chamoun NG. Broadly applicable risk stratification system for predicting duration of hospitalization and mortality. Anesthesiology. 2010; 113:1026–37. DOI: 10.1097/ALN.0b013e3181f79a8d. PMID: 20966661.16. Chamoun GF, Li L, Chamoun NG, Saini V, Sessler DI. Validation and calibration of the risk stratification index. Anesthesiology. 2017; 126:623–30. DOI: 10.1097/ALN.0000000000001560. PMID: 28187023.17. Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016; 3:160035. DOI: 10.1038/sdata.2016.35. PMID: 27219127. PMCID: PMC4878278.18. Lee HC, Jung CW. Vital recorder-a free research tool for automatic recording of high-resolution time-synchronised physiological data from multiple anaesthesia devices. Sci Rep. 2018; 8:1527. DOI: 10.1038/s41598-018-20062-4. PMID: 29367620. PMCID: PMC5784161.19. Ioffe S, Szegedy C. Batch normalization: accelerating deep network training by reducing internal covariate shift. Paper presented at:32nd International Conference on Machine Learning. 2015; Jul. 7-9. Lille, France. arXiv preprint arXiv: 150203167.20. Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974; 19:716–23. DOI: 10.1109/TAC.1974.1100705.21. Stone M. Cross-validatory choice and assessment of statistical predictions. J R Stat Soc. Ser B (Methodological). 1974; 36:111–47.22. Rumelhart DE, Widrow B, Lehr MA. The basic ideas in neural networks. Commun ACM. 1994; 37:87–92. DOI: 10.1145/175247.175256.23. Cortes C, Vapnik V. Support-vector networks. Mach Learn. 1995; 20:273–97. DOI: 10.1023/A:1022627411411.24. Chawla NV, Bowyer KW, Hall LO, Kegelmeyer WP. SMOTE: synthetic minority over-sampling technique. J Artifi Intell Res. 2002; 16:321–57. DOI: 10.1613/jair.953.25. Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Ser B (Methodological). 1996; 58:267–88.26. Fischler MA, Bolles RC. Random sample consensus: a paradigm for model fitting with applications to image analysis and automated cartography. Commun ACM. 1981; 24:381–95. DOI: 10.1145/358669.358692.27. Breiman L. Bagging predictors. Mach Learn. 1996; 24:123–40. DOI: 10.1007/BF00058655.28. Freund Y. Boosting a weak learning algorithm by majority. Inf Computat. 1995; 121:256–85. DOI: 10.1006/inco.1995.1136.29. Breiman L. Random forests. Mach Learn. 2001; 45:5–32. DOI: 10.1023/A:1010933404324.30. Fernández-Delgado M, Cernadas E, Barro S, Amorim D. Do we need hundreds of classifiers to solve real world classification problems? J Mach Learn Res. 2014; 15:3133–81.31. Desautels T, Calvert J, Hoffman J, Jay M, Kerem Y, Shieh L, et al. Prediction of sepsis in the intensive care unit with minimal electronic health record data: a machine learning approach. JMIR Med Inform. 2016; 4:e28. DOI: 10.2196/medinform.5909. PMID: 27694098. PMCID: PMC5065680.32. Mao Q, Jay M, Hoffman JL, Calvert J, Barton C, Shimabukuro D, et al. Multicentre validation of a sepsis prediction algorithm using only vital sign data in the emergency department, general ward and ICU. BMJ Open. 2018; 8:e017833. DOI: 10.1136/bmjopen-2017-017833. PMID: 29374661. PMCID: PMC5829820.33. Koyner JL, Carey KA, Edelson DP, Churpek MM. The development of a machine learning inpatient acute kidney injury prediction model. Crit Care Med. 2018; 46:1070–7. DOI: 10.1097/CCM.0000000000003123. PMID: 29596073.34. McCulloch WS, Pitts W. A logical calculus of the ideas immanent in nervous activity. Bull Math Biol. 1990; 52:99–115. discussion 73-97. DOI: 10.1007/BF02459570. DOI: 10.1016/S0092-8240(05)80006-0.35. Hinton GE, Osindero S, Teh YW. A fast learning algorithm for deep belief nets. Neural Comput. 2006; 18:1527–54. DOI: 10.1162/neco.2006.18.7.1527. PMID: 16764513.36. Srivastava N, Hinton G, Krizhevsky A, Sutskever I, Salakhutdinov R. Dropout: a simple way to prevent neural networks from overfitting. J Mach Learn Res. 2014; 15:1929–58.37. Nair V, Hinton GE. Rectified linear units improve restricted boltzmann machines. Paper presented at: the 27th international conference on machine learning. 2010; Jun. 21-24. Haifa, Israel. Paper ID:432.38. Lee CK, Hofer I, Gabel E, Baldi P, Cannesson M. Development and validation of a deep neural network model for prediction of postoperative in-hospital mortality. Anesthesiology. 2018; doi:10.1097/ALN.0000000000002186. [Epub ahead of print]. DOI: 10.1097/ALN.0000000000002186.39. Lecun Y, Bottou L, Bengio Y, Haffner P. Gradient-based learning applied to document recognition. Proc IEEE. 1998; 86:2278–324. DOI: 10.1109/5.726791.40. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017; 284:574–82. DOI: 10.1148/radiol.2017162326. PMID: 28436741.41. Rajpurkar P, Hannun AY, Haghpanahi M, Bourn C, Ng AY. Cardiologist-level arrhythmia detection with convolutional neural networks arXiv.org [serial on the Internet]. 2017. [2018 Jun 5]. Available from https://arxiv.org/abs/1707.01836.42. Hochreiter S, Schmidhuber J. Long short-term memory. Neural Comput. 1997; 9:1735–80. DOI: 10.1162/neco.1997.9.8.1735. PMID: 9377276.43. Chung J, Gulcehre C, Cho KH, Bengio Y. Empirical evaluation of gated recurrent neural networks on sequence modeling arXiv.org [serial on the Internet]. 2014. [2018 Jun 5]. Available from https://arxiv.org/abs/1412.3555.44. Lee HC, Ryu HG, Chung EJ, Jung CW. Prediction of bispectral index during target-controlled infusion of propofol and remifentanil: a deep learning approach. Anesthesiology. 2018; 128:492–501. DOI: 10.1097/ALN.0000000000001892. PMID: 28953500.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of a Diabetologist in the New Era of Artificial Intelligence
- The Role of medical doctor in the era of artificial intelligence
- Artificial Intelligence in Pathology
- Application of artificial intelligence for diagnosis of early gastric cancer based on magnifying endoscopy with narrow-band imaging
- Artificial Intelligence for Improved Patient Outcomes—The Pragmatic Randomized Controlled Trial Is the Secret Sauce