J Korean Neurosurg Soc.
2019 Jan;62(1):3-9. 10.3340/jkns.2018.0151.
Variability of Platelet Reactivity on Antiplatelet Therapy in Neurointervention Procedure
- Affiliations
-
- 1Department of Neurosurgery, Hangang Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea. storynlemon@gmail.com
- KMID: 2434341
- DOI: http://doi.org/10.3340/jkns.2018.0151
Abstract
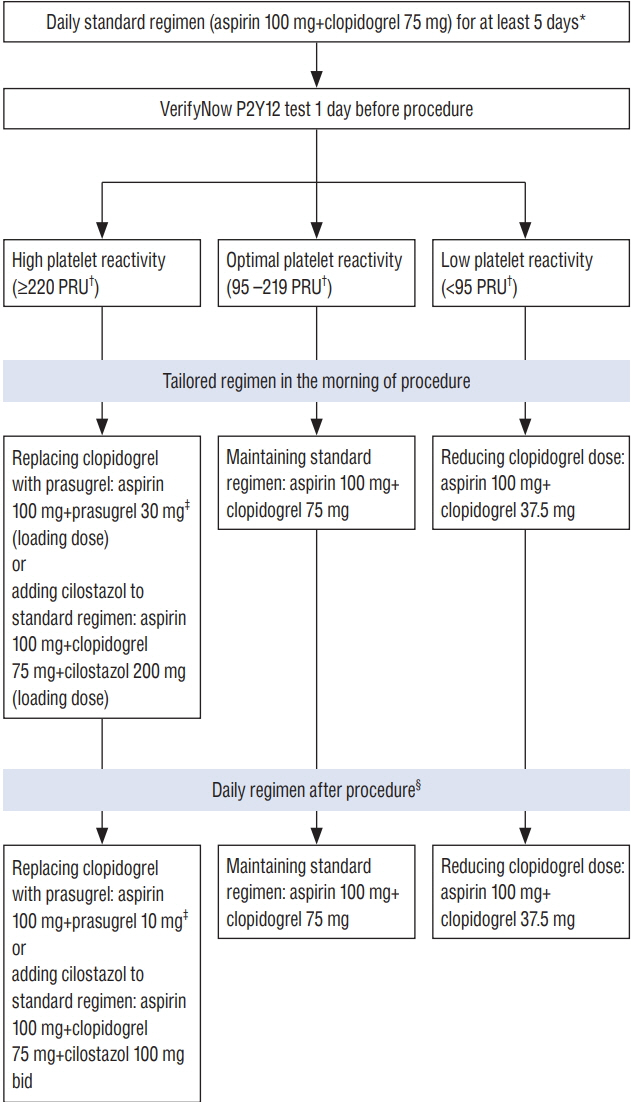
- As more intracranial aneurysms and other cerebrovascular pathologies are treated with neurointervention procedure, thromboembolic events that frequently lead to serious neurological deficit or fatal outcomes are increasing. In order to prevent the thromboembolic events, antiplatelet therapy is used in most procedures including coil embolization, stenting, and flow diversion. However, because of variable individual pharmacodynamics responses to antiplatelet drugs, especially clopidogrel, it is difficult for clinicians to select the adequate antiplatelet regimen and its optimal dose. This article reviews the neurointervention literature related to antiplatelet therapy and suggests a strategy for tailoring antiplatelet therapy in individual patients undergoing neurointervention based on the results of platelet function testing.
MeSH Terms
Figure
Cited by 1 articles
-
Preliminary Experience of Neuroform Atlas Stenting as a Rescue Treatment after Failure of Mechanical Thrombectomy Caused by Residual Intracranial Atherosclerotic Stenosis
Ho Jun Yi, Jae Hoon Sung, Dong Hoon Lee
J Korean Neurosurg Soc. 2021;64(2):198-206. doi: 10.3340/jkns.2020.0146.
Reference
-
References
1. Akbari SH, Reynolds MR, Kadkhodayan Y, Cross DT 3rd, Moran CJ. Hemorrhagic complications after prasugrel (Effient) therapy for vascular neurointerventional procedures. J Neurointerv Surg. 5:337–343. 2013.
Article2. Aradi D, Collet JP, Mair J, Plebani M, Merkely B, Jaffe AS, et al. Platelet function testing in acute cardiac care - is there a role for prediction or prevention of stent thrombosis and bleeding? Thromb Haemost. 113:221–230. 2015.
Article3. Aradi D, Kirtane A, Bonello L, Gurbel PA, Tantry US, Huber K, et al. Bleeding and stent thrombosis on P2Y12-inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J. 36:1762–1771. 2015.
Article4. Asai T, Miyachi S, Izumi T, Matsubara N, Haraguchi K, Yamanouchi T, et al. Relationship between low response to clopidogrel and periprocedural ischemic events with coil embolization for intracranial aneurysms. J Neurointerv Surg. 8:752–755. 2016.
Article5. Buch AN, Singh S, Roy P, Javaid A, Smith KA, George CE, et al. Measuring aspirin resistance, clopidogrel responsiveness, and postprocedural markers of myonecrosis in patients undergoing percutaneous coronary intervention. Am J Cardiol. 99:1518–1522. 2007.
Article6. Cattaneo M. Laboratory detection of 'aspirin resistance': what test should we use (if any)? Eur Heart J. 28:1673–1675. 2007.
Article7. Chalouhi N, Tjoumakaris S, Dumont AS, Gonzalez LF, Randazzo C, Starke RM, et al. Treatment of posterior circulation aneurysms with the pipeline embolization device. Neurosurgery. 72:883–889. 2013.
Article8. Choi HH, Lee JJ, Cho YD, Han MH, Cho WS, Kim JE, et al. Antiplatelet premedication for stent-assisted coil embolization of intracranial aneurysms: low-dose prasugrel vs clopidogrel. Neurosurgery. 83:981–988. 2018.
Article9. Daou B, Starke RM, Chalouhi N, Barros G, Tjoumakaris S, Rosenwasser RH, et al. P2Y12 reaction units: effect on hemorrhagic and thromboembolic complications in patients with cerebral aneurysms treated with the pipeline embolization device. Neurosurgery. 78:27–33. 2016.10. Delgado Almandoz JE, Crandall BM, Scholz JM, Fease JL, Anderson RE, Kadkhodayan Y, et al. Pre-procedure P2Y12 reaction units value predicts perioperative thromboembolic and hemorrhagic complications in patients with cerebral aneurysms treated with the pipeline embolization device. J Neurointerv Surg. 5 Suppl 3:iii3–iii10. 2013.
Article11. Douketis JD, Berger PB, Dunn AS, Jaffer AK, Spyropoulos AC, Becker RC, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). 6 Suppl:299S–339S. 2008.12. Drazin D, Choulakian A, Nuño M, Kornbluth P, Alexander MJ. Body weight: a risk factor for subtherapeutic antithrombotic therapy in neurovascular stenting. J Neurointerv Surg. 3:177–181. 2011.
Article13. Farid NA, Payne CD, Small DS, Winters KJ, Ernest CS 2nd, Brandt JT, et al. Cytochrome P450 3A inhibition by ketoconazole affects prasugrel and clopidogrel pharmacokinetics and pharmacodynamics differently. Clin Pharmacol Ther. 81:735–741. 2007.
Article14. Fifi JT, Brockington C, Narang J, Leesch W, Ewing SL, Bennet H, et al. Clopidogrel resistance is associated with thromboembolic complications in patients undergoing neurovascular stenting. AJNR Am J Neuroradiol. 34:716–720. 2013.
Article15. Gaglia MA, Torguson R, Pakala R, Xue Z, Sardi G, Suddath WO, et al. Correlation between light transmission aggregometry, VerifyNow P2Y12, and VASP-P platelet reactivity assays following percutaneous coronary intervention. J Interv Cardiol. 24:529–534. 2011.
Article16. Godino C, Mendolicchio L, Figini F, Latib A, Sharp AS, Cosgrave J, et al. Comparison of VerifyNow-P2Y12 test and flow cytometry for monitoring individual platelet response to clopidogrel. What is the cut-off value for identifying patients who are low responders to clopidogrel therapy? Thromb J. 7:4. 2009.
Article17. Goh C, Churilov L, Mitchell P, Dowling R, Yan B. Clopidogrel hyperresponse and bleeding risk in neurointerventional procedures. AJNR Am J Neuroradiol. 34:721–726. 2013.
Article18. Gross L, Aradi D, Sibbing D. Platelet function testing in patients on antiplatelet medications. Semin Thromb Hemost. 42:306–320. 2016.
Article19. Ha EJ, Cho WS, Kim JE, Cho YD, Choi HH, Kim T, et al. Prophylactic antiplatelet medication in endovascular treatment of intracranial aneurysms: low-dose prasugrel versus clopidogrel. AJNR Am J Neuroradiol. 37:2060–2065. 2016.
Article20. Hanel RA, Taussky P, Dixon T, Miller DA, Sapin M, Nordeen JD, et al. Safety and efficacy of ticagrelor for neuroendovascular procedures. A single center initial experience. J Neurointerv Surg. 6:320–322. 2014.
Article21. Hwang G, Huh W, Lee JS, Villavicencio JB, Villamor RB Jr, Ahn SY, et al. Standard vs modified antiplatelet preparation for preventing thromboembolic events in patients with high on-treatment platelet reactivity undergoing coil embolization for an unruptured intracranial aneurysm: a randomized clinical trial. JAMA Neurol. 72:764–772. 2015.
Article22. Kang HS, Kwon BJ, Kim JE, Han MH. Preinterventional clopidogrel response variability for coil embolization of intracranial aneurysms: clinical implications. AJNR Am J Neuroradiol. 31:1206–1210. 2010.
Article23. Kashiwazaki D, Kuwayama N, Akioka N, Hayakawa Y, Kuroda S. The roles and issues of P2Y12 percent inhibition assessed by VerifyNow assay for patients undergoing Neurointervention: a prospective study. J Stroke Cerebrovasc Dis. 23:1830–1836. 2014.
Article24. Kim B, Kim K, Jeon P, Kim S, Kim H, Byun H, et al. Thromboembolic complications in patients with clopidogrel resistance after coil embolization for unruptured intracranial aneurysms. AJNR Am J Neuroradiol. 35:1786–1792. 2014.
Article25. Kim CH, Hwang G, Kwon OK, Ban SP, Chinh ND, Tjahjadi M, et al. P2Y12 reaction units threshold for implementing modified antiplatelet preparation in coil embolization of unruptured aneurysms: a prospective validation study. Radiology. 282:542–551. 2017.
Article26. Kim MH, Zhang HZ, Jung DK. Pharmacodynamic comparisons for single loading doses of prasugrel (30 mg) and clopidogrel (600 mg) in healthy Korean volunteers. Circ J. 77:1253–1259. 2013.
Article27. Koerner H, Derveaux C, Alexandrou M, Graeber S, Roth C, Papanagiotou P, et al. Do clopidogrel nonresponders have an increased risk of adverse events during supra-aortal angioplasty and stenting? Stroke Res Treat. 2012:904534. 2012.
Article28. Kovács EG, Katona É, Bereczky Z, Homoródi N, Balogh L, Tóth E, et al. Evaluation of laboratory methods routinely used to detect the effect of aspirin against new reference methods. Thromb Res. 133:811–816. 2014.
Article29. Kovács EG, Katona É, Bereczky Z, Homoródi N, Balogh L, Tóth E, et al. New direct and indirect methods for the detection of cyclooxygenase 1 acetylation by aspirin; the lack of aspirin resistance among healthy individuals. Thromb Res. 131:320–324. 2013.
Article30. Lee DH, Arat A, Morsi H, Shaltoni H, Harris JR, Mawad ME. Dual antiplatelet therapy monitoring for neurointerventional procedures using a point-of-care platelet function test: a single-center experience. AJNR Am J Neuroradiol. 29:1389–1394. 2008.
Article31. Lee DH, Kim MH, Guo LZ, Park MK, Yi SJ. Lower loading dose of prasugrel compared with conventional loading doses of clopidogrel and prasugrel in Korean patients undergoing elective coronary angiography: a randomized controlled study evaluating pharmacodynamic efficacy. Korean Circ J. 44:386–393. 2014.
Article32. Michelson AD, Cattaneo M, Eikelboom JW, Gurbel P, Kottke-Marchant K, Kunicki TJ, et al. Aspirin resistance: position paper of the working group on aspirin resistance. J Thromb Haemost. 3:1309–1311. 2005.
Article33. Nishi H, Nakahara I, Matsumoto S, Hashimoto T, Ohta T, Sadamasa N, et al. Platelet reactivity and hemorrhage risk in neurointerventional procedures under dual antiplatelet therapy. J Neurointerv Surg. 8:949–953. 2016.
Article34. Nordeen JD, Patel AV, Darracott RM, Johns GS, Taussky P, Tawk RG, et al. Clopidogrel resistance by P2Y12 platelet function testing in patients undergoing neuroendovascular procedures: incidence of ischemic and hemorrhagic complications. J Vasc Interv Neurol. 6:26–34. 2013.35. Pandya DJ, Fitzsimmons BF, Wolfe TJ, Hussain SI, Lynch JR, Ortega-Gutierrez S, et al. Measurement of antiplatelet inhibition during neurointerventional procedures: the effect of antithrombotic duration and loading dose. J Neuroimaging. 20:64–69. 2010.
Article36. Paniccia R, Priora R, Liotta AA, Abbate R. Platelet function tests: a comparative review. Vasc Health Risk Manag. 11:133–148. 2015.
Article37. Paré G, Mehta SR, Yusuf S, Anand SS, Connolly SJ, Hirsh J, et al. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N Engl J Med. 363:1704–1714. 2010.
Article38. Patrono C, García Rodríguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 353:2373–2383. 2005.
Article39. Pierot L, Wakhloo AK. Endovascular treatment of intracranial aneurysms: current status. Stroke. 44:2046–2054. 2013.40. Prabhakaran S, Wells KR, Lee VH, Flaherty CA, Lopes DK. Prevalence and risk factors for aspirin and clopidogrel resistance in cerebrovascular stenting. AJNR Am J Neuroradiol. 29:281–285. 2008.
Article41. Scott SA, Sangkuhl K, Shuldiner AR, Hulot JS, Thorn CF, Altman RB, et al. PharmGKB summary: very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 19. Pharmacogenet Genomics. 22:159–165. 2012.42. Simon T, Steg PG, Gilard M, Blanchard D, Bonello L, Hanssen M, et al. Clinical events as a function of proton pump inhibitor use, clopidogrel use, and cytochrome P450 2C19 genotype in a large nationwide cohort of acute myocardial infarction: results from the French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) registry. Circulation. 123:474–482. 2011.
Article43. Stetler WR, Chaudhary N, Thompson BG, Gemmete JJ, Maher CO, Pandey AS. Prasugrel is effective and safe for neurointerventional procedures. J Neurointerv Surg. 5:332–336. 2013.
Article44. Tan LA, Keigher KM, Munich SA, Moftakhar R, Lopes DK. Thromboembolic complications with pipeline embolization device placement: impact of procedure time, number of stents and pre-procedure P2Y12 reaction unit (PRU) value. J Neurointerv Surg. 7:217–221. 2015.
Article45. Tantry US, Bonello L, Aradi D, Price MJ, Jeong YH, Angiolillo DJ, et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 62:2261–2273. 2013.
Article46. Yu KS, Park KW, Kelly RP, Gu N, Payne C, Small DS, et al. Pharmacokinetic and pharmacodynamic effects of prasugrel in healthy Korean males. J Cardiovasc Pharmacol. 62:72–77. 2013.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Change of Platelet Reactivity to Antiplatelet Therapy after Stenting Procedure for Cerebral Artery Stenosis: VerifyNow Antiplatelet Assay before and after Stenting
- Temporal Variability of Platelet Reactivity in Patients Treated with Clopidogrel or Ticagrelor
- Effects of Platelet Number and Platelet Indices on Platelet Reactivity in Patients Treated with Clopidogrel or Ticagrelor
- Temporal Variability of Platelet Reactivity Phenotype: Another Barrier to Personalized Antiplatelet Strategy Guided by Platelet Function Testing
- Anti-Platelet Drug Resistance in the Prediction of Thromboembolic Complications after Neurointervention