Nutr Res Pract.
2019 Feb;13(1):3-10. 10.4162/nrp.2019.13.1.3.
Nicotinamide riboside regulates inflammation and mitochondrial markers in AML12 hepatocytes
- Affiliations
-
- 1Department of Food and Nutrition, Seoul Women's University, 621 Hwarangro, Nowon-Gu, Seoul 01797, Korea. sjyang89@swu.ac.kr
- KMID: 2434070
- DOI: http://doi.org/10.4162/nrp.2019.13.1.3
Abstract
- BACKGROUND/OBJECTIVES
The NAD+ precursor nicotinamide riboside (NR) is a type of vitamin B3 found in cow's milk and yeast-containing food products such as beer. Recent studies suggested that NR prevents hearing loss, high-fat diet-induced obesity, Alzheimer's disease, and mitochondrial myopathy. The objective of this study was to investigate the effects of NR on inflammation and mitochondrial biogenesis in AML12 mouse hepatocytes.
MATERIALS/METHODS
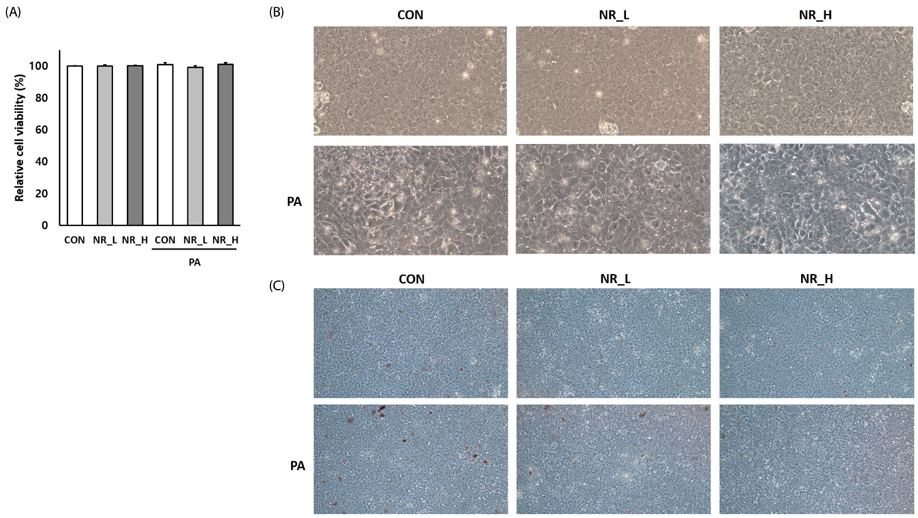
A subset of hepatocytes was treated with palmitic acid (PA; 250 µM) for 48 h to induce hepatocyte steatosis. The hepatocytes were treated with NR (10 µM and 10 mM) for 24 h with and without PA. The cell viability and the levels of sirtuins, inflammatory markers, and mitochondrial markers were analyzed.
RESULTS
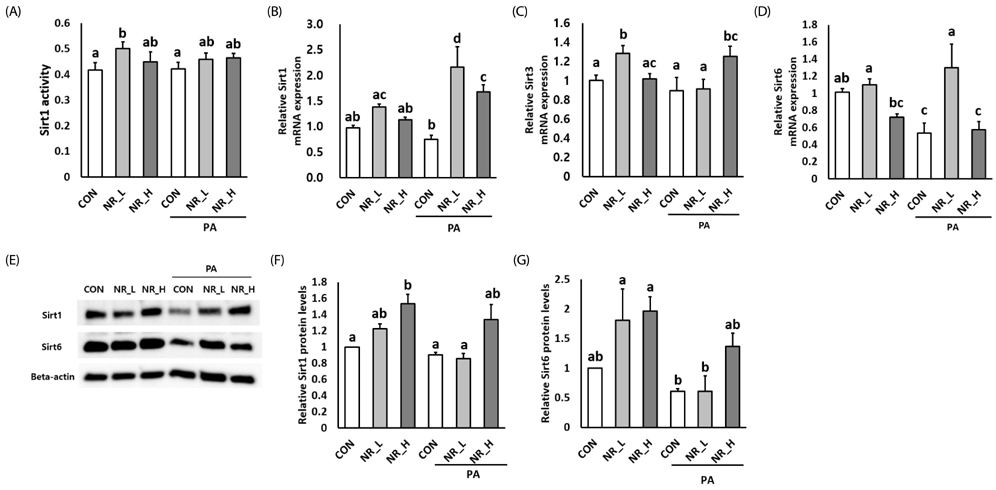
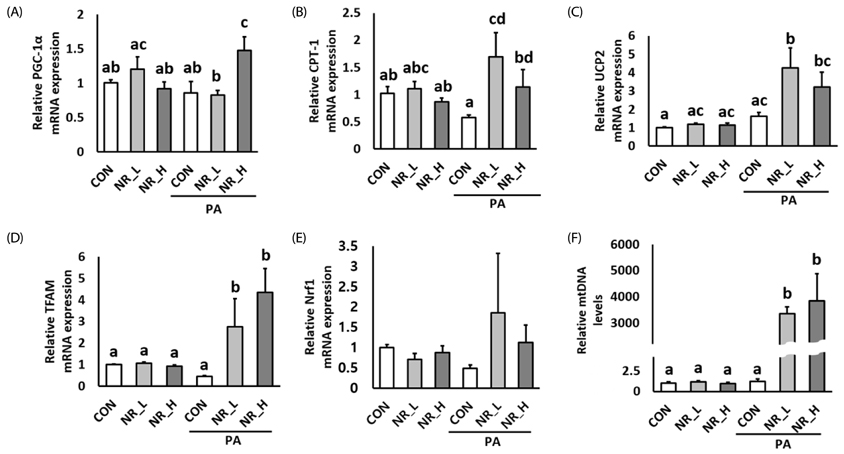
Cytotoxicity of NR was examined by PrestoBlue assay. Exposure to NR had no effect on cell viability or morphology. Gene expression of sirtuin 1 (Sirt1) and Sirt3 was significantly upregulated by NR in PA-treated hepatocytes. However, Sirt1 activities were increased in hepatocytes treated with low-dose NR. Hepatic pro-inflammatory markers including tumor necrosis factor-alpha and interleukin-6 were decreased in NR-treated cells. NR upregulated anti-inflammatory molecule adiponectin, and, tended to down-regulate hepatokine fetuin-A in PA-treated hepatocytes, suggesting its inverse regulation on these cytokines. NR increased levels of mitochondrial markers including peroxisome proliferator-activated receptor γ coactivator-1α, carnitine palmitoyltransferase 1, uncoupling protein 2, transcription factor A, mitochondrial and mitochondrial DNA in PA-treated hepatocytes.
CONCLUSIONS
These data demonstrated that NR attenuated hepatic inflammation and increased levels of mitochondrial markers in hepatocytes.
Keyword
MeSH Terms
-
Adiponectin
alpha-2-HS-Glycoprotein
Alzheimer Disease
Animals
Beer
Carnitine O-Palmitoyltransferase
Cell Survival
Cytokines
DNA, Mitochondrial
Fatty Liver
Gene Expression
Hearing Loss
Hepatocytes*
Inflammation*
Interleukin-6
Mice
Milk
Mitochondria
Mitochondrial Myopathies
Niacin
Niacinamide*
Obesity
Organelle Biogenesis
Palmitic Acid
Peroxisomes
Sirtuin 1
Sirtuins
Transcription Factors
Tumor Necrosis Factor-alpha
Adiponectin
Carnitine O-Palmitoyltransferase
Cytokines
DNA, Mitochondrial
Interleukin-6
Niacin
Niacinamide
Palmitic Acid
Sirtuin 1
Sirtuins
Transcription Factors
Tumor Necrosis Factor-alpha
alpha-2-HS-Glycoprotein
Figure
Reference
-
1. Trammell SA, Yu L, Redpath P, Migaud ME, Brenner C. Nicotinamide riboside is a major NAD+ precursor vitamin in cow milk. J Nutr. 2016; 146:957–963.
Article2. Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell. 2007; 129:473–484.
Article3. Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004; 117:495–502.
Article4. Julius U. Niacin as antidyslipidemic drug. Can J Physiol Pharmacol. 2015; 93:1043–1054.
Article5. Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008; 28:115–130.
Article6. Yang SJ, Choi JM, Kim L, Park SE, Rhee EJ, Lee WY, Oh KW, Park SW, Park CY. Nicotinamide improves glucose metabolism and affects the hepatic NAD-sirtuin pathway in a rodent model of obesity and type 2 diabetes. J Nutr Biochem. 2014; 25:66–72.
Article7. Shima K, Zhu M, Kuwajima M. A role of nicotinamide-induced increase in pancreatic β-cell mass on blood glucose control after discontinuation of the treatment in partially pancreatectomized OLETF rats. Diabetes Res Clin Pract. 1998; 41:1–8.
Article8. Knip M, Douek IF, Moore WP, Gillmor HA, McLean AE, Bingley PJ, Gale EA. Safety of high-dose nicotinamide: a review. Diabetologia. 2000; 43:1337–1345.
Article9. Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016; 7:12948.
Article10. Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012; 15:838–847.
Article11. Brown KD, Maqsood S, Huang JY, Pan Y, Harkcom W, Li W, Sauve A, Verdin E, Jaffrey SR. Activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 2014; 20:1059–1068.
Article12. Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsström S, Pasila L, Velagapudi V, Carroll CJ, Auwerx J, Suomalainen A. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol Med. 2014; 6:721–731.
Article13. Trammell SA, Weidemann BJ, Chadda A, Yorek MS, Holmes A, Coppey LJ, Obrosov A, Kardon RH, Yorek MA, Brenner C. Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci Rep. 2016; 6:26933.
Article14. Gariani K, Menzies KJ, Ryu D, Wegner CJ, Wang X, Ropelle ER, Moullan N, Zhang H, Perino A, Lemos V, Kim B, Park YK, Piersigilli A, Pham TX, Yang Y, Ku CS, Koo SI, Fomitchova A, Cantó C, Schoonjans K, Sauve AA, Lee JY, Auwerx J. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology. 2016; 63:1190–1204.
Article15. Lee HJ, Hong YS, Jun W, Yang SJ. Nicotinamide riboside ameliorates hepatic metaflammation by modulating NLRP3 inflammasome in a rodent model of type 2 diabetes. J Med Food. 2015; 18:1207–1213.
Article16. Mukherjee S, Chellappa K, Moffitt A, Ndungu J, Dellinger RW, Davis JG, Agarwal B, Baur JA. Nicotinamide adenine dinucleotide biosynthesis promotes liver regeneration. Hepatology. 2017; 65:616–630.
Article17. Zhou CC, Yang X, Hua X, Liu J, Fan MB, Li GQ, Song J, Xu TY, Li ZY, Guan YF, Wang P, Miao CY. Hepatic NAD+ deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br J Pharmacol. 2016; 173:2352–2368.
Article18. Stafeev IS, Menshikov MY, Tsokolaeva ZI, Shestakova MV, Parfyonova YV. Molecular mechanisms of latent inflammation in metabolic syndrome. possible role of sirtuins and peroxisome proliferator-activated receptor type γ. Biochemistry (Mosc). 2015; 80:1217–1226.
Article19. Mendes KL, Lelis DF, Santos SH. Nuclear sirtuins and inflammatory signaling pathways. Cytokine Growth Factor Rev. 2017; 38:98–105.
Article20. Ding RB, Bao J, Deng CX. Emerging roles of SIRT1 in fatty liver diseases. Int J Biol Sci. 2017; 13:852–867.
Article21. Liu P, Huang G, Wei T, Gao J, Huang C, Sun M, Zhu L, Shen W. Sirtuin 3-induced macrophage autophagy in regulating NLRP3 inflammasome activation. Biochim Biophys Acta Mol Basis Dis. 2018; 1864:764–777.
Article22. Paulin R, Dromparis P, Sutendra G, Gurtu V, Zervopoulos S, Bowers L, Haromy A, Webster L, Provencher S, Bonnet S, Michelakis ED. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metab. 2014; 20:827–839.
Article23. Gleave JA, Arathoon LR, Trinh D, Lizal KE, Giguère N, Barber JH, Najarali Z, Khan MH, Thiele SL, Semmen MS, Koprich JB, Brotchie JM, Eubanks JH, Trudeau LE, Nash JE. Sirtuin 3 rescues neurons through the stabilisation of mitochondrial biogenetics in the virally-expressing mutant α-synuclein rat model of parkinsonism. Neurobiol Dis. 2017; 106:133–146.
Article24. Li Y, Ye Z, Lai W, Rao J, Huang W, Zhang X, Yao Z, Lou T. Activation of sirtuin 3 by silybin attenuates mitochondrial dysfunction in cisplatin-induced acute kidney injury. Front Pharmacol. 2017; 8:178.
Article25. Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004; 306:2105–2108.
Article26. Noriega LG, Feige JN, Canto C, Yamamoto H, Yu J, Herman MA, Mataki C, Kahn BB, Auwerx J. CREB and ChREBP oppositely regulate SIRT1 expression in response to energy availability. EMBO Rep. 2011; 12:1069–1076.
Article27. Hayashida S, Arimoto A, Kuramoto Y, Kozako T, Honda S, Shimeno H, Soeda S. Fasting promotes the expression of SIRT1, an NAD+-dependent protein deacetylase, via activation of PPARα in mice. Mol Cell Biochem. 2010; 339:285–292.
Article28. Okazaki M, Iwasaki Y, Nishiyama M, Taguchi T, Tsugita M, Nakayama S, Kambayashi M, Hashimoto K, Terada Y. PPARβ/δ regulates the human SIRT1 gene transcription via Sp1. Endocr J. 2010; 57:403–413.
Article29. Han L, Zhou R, Niu J, McNutt MA, Wang P, Tong T. SIRT1 is regulated by a PPARγ-SIRT1 negative feedback loop associated with senescence. Nucleic Acids Res. 2010; 38:7458–7471.
Article30. Sasaki T, Maier B, Koclega KD, Chruszcz M, Gluba W, Stukenberg PT, Minor W, Scrable H. Phosphorylation regulates SIRT1 function. PLoS One. 2008; 3:e4020.
Article31. Nasrin N, Kaushik VK, Fortier E, Wall D, Pearson KJ, de Cabo R, Bordone L. JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS One. 2009; 4:e8414.
Article32. Guo X, Williams JG, Schug TT, Li X. DYRK1A and DYRK3 promote cell survival through phosphorylation and activation of SIRT1. J Biol Chem. 2010; 285:13223–13232.
Article33. Yang Y, Fu W, Chen J, Olashaw N, Zhang X, Nicosia SV, Bhalla K, Bai W. SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat Cell Biol. 2007; 9:1253–1262.
Article34. Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell. 2007; 28:277–290.
Article35. Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature. 2004; 429:771–776.
Article36. Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008; 451:583–586.
Article37. Zhao W, Kruse JP, Tang Y, Jung SY, Qin J, Gu W. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008; 451:587–590.
Article38. Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012; 13:225–238.
Article39. Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011; 332:1519–1523.
Article40. Kinoshita T, Imamura R, Kushiyama H, Suda T. NLRP3 mediates NF-κ B activation and cytokine induction in microbially induced and sterile inflammation. PLoS One. 2015; 10:e0119179.41. Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001; 107:7–11.
Article42. Lin X, Braymer HD, Bray GA, York DA. Differential expression of insulin receptor tyrosine kinase inhibitor (fetuin) gene in a model of diet-induced obesity. Life Sci. 1998; 63:145–153.
Article43. Lee HJ, Lim Y, Yang SJ. Involvement of resveratrol in crosstalk between adipokine adiponectin and hepatokine fetuin-A in vivo and in vitro. J Nutr Biochem. 2015; 26:1254–1260.
Article44. Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006; 6:1–28.
Article45. Sheng B, Wang X, Su B, Lee HG, Casadesus G, Perry G, Zhu X. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer's disease. J Neurochem. 2012; 120:419–429.
Article46. Ren J, Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med (Berl). 2010; 88:993–1001.
Article47. López-Lluch G, Irusta PM, Navas P, de Cabo R. Mitochondrial biogenesis and healthy aging. Exp Gerontol. 2008; 43:813–819.
Article48. Austin S, St-Pierre J. PGC1α and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci. 2012; 125:4963–4971.
Article49. Goffart S, Wiesner RJ. Regulation and co-ordination of nuclear gene expression during mitochondrial biogenesis. Exp Physiol. 2003; 88:33–40.
Article50. Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006; 27:728–735.
Article51. Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA, Pasinetti GM. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer's mouse models. Neurobiol Aging. 2013; 34:1581–1588.
Article52. Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011; 93:884S–890S.
Article53. Aharoni-Simon M, Hann-Obercyger M, Pen S, Madar Z, Tirosh O. Fatty liver is associated with impaired activity of PPARγ-coactivator 1α (PGC1α) and mitochondrial biogenesis in mice. Lab Invest. 2011; 91:1018–1028.
Article54. Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006; 116:615–622.
Article55. Puigserver P. Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-α. Int J Obes (Lond). 2005; 29:Suppl 1. S5–S9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rejuvenating Aged Hematopoietic Stem Cells Through Improvement of Mitochondrial Function
- The Relationship between Mitochondria and NLRP3 Inflammasome
- Mitochondrial Quality Control: Its Role in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
- Impairment of Mitochondrial ATP Synthesis Induces RIPK3-dependent Necroptosis in Lung Epithelial Cells During Lung Injury by Lung Inflammation
- A Novel Nicotinamide Adenine Dinucleotide Correction Method for Mitochondrial Ca2+ Measurement with FURA-2-FF in Single Permeabilized Ventricular Myocytes of Rat