Nat Prod Sci.
2018 Dec;24(4):288-292. 10.20307/nps.2018.24.4.288.
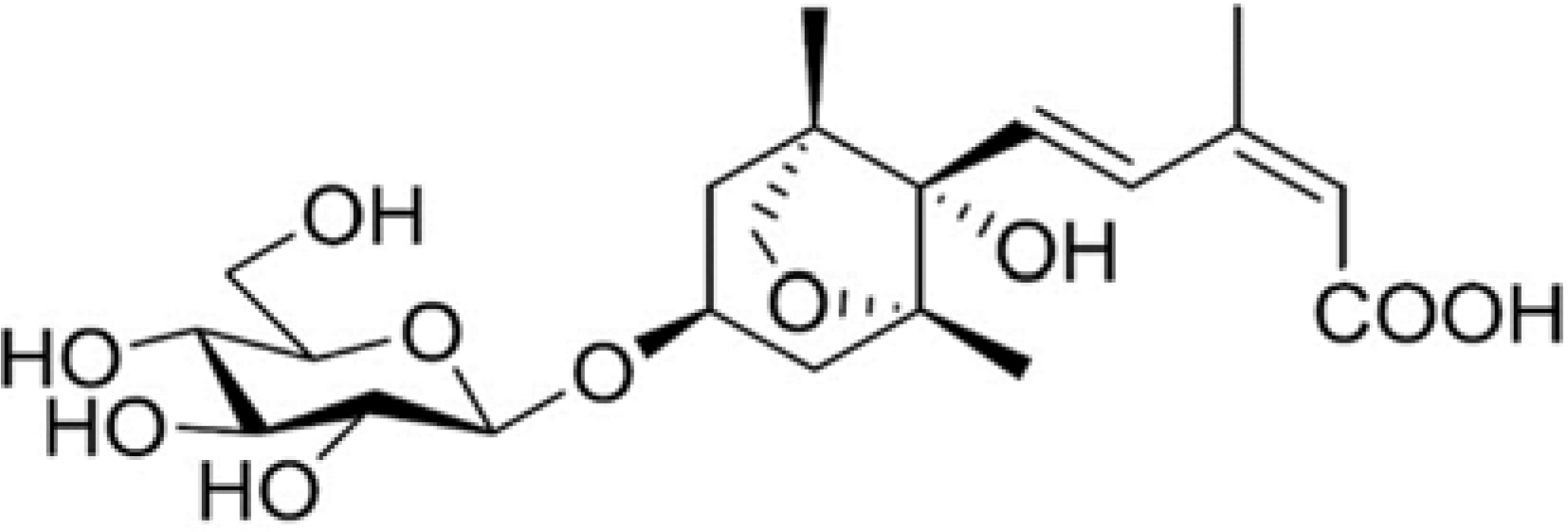
Efficient Isolation of Dihydrophaseic acid 3′-O-β-D-Glucopyranoside from Nelumbo nucifera Seeds Using High-performance Countercurrent Chromatography and Reverse-phased High-performance Liquid Chromatography
- Affiliations
-
- 1College of Pharmacy and Integrated Research Institute of Pharmaceutical Sciences, The Catholic University of Korea, Bucheon14662, Korea. kdyoon@catholic.ac.kr
- KMID: 2432425
- DOI: http://doi.org/10.20307/nps.2018.24.4.288
Abstract
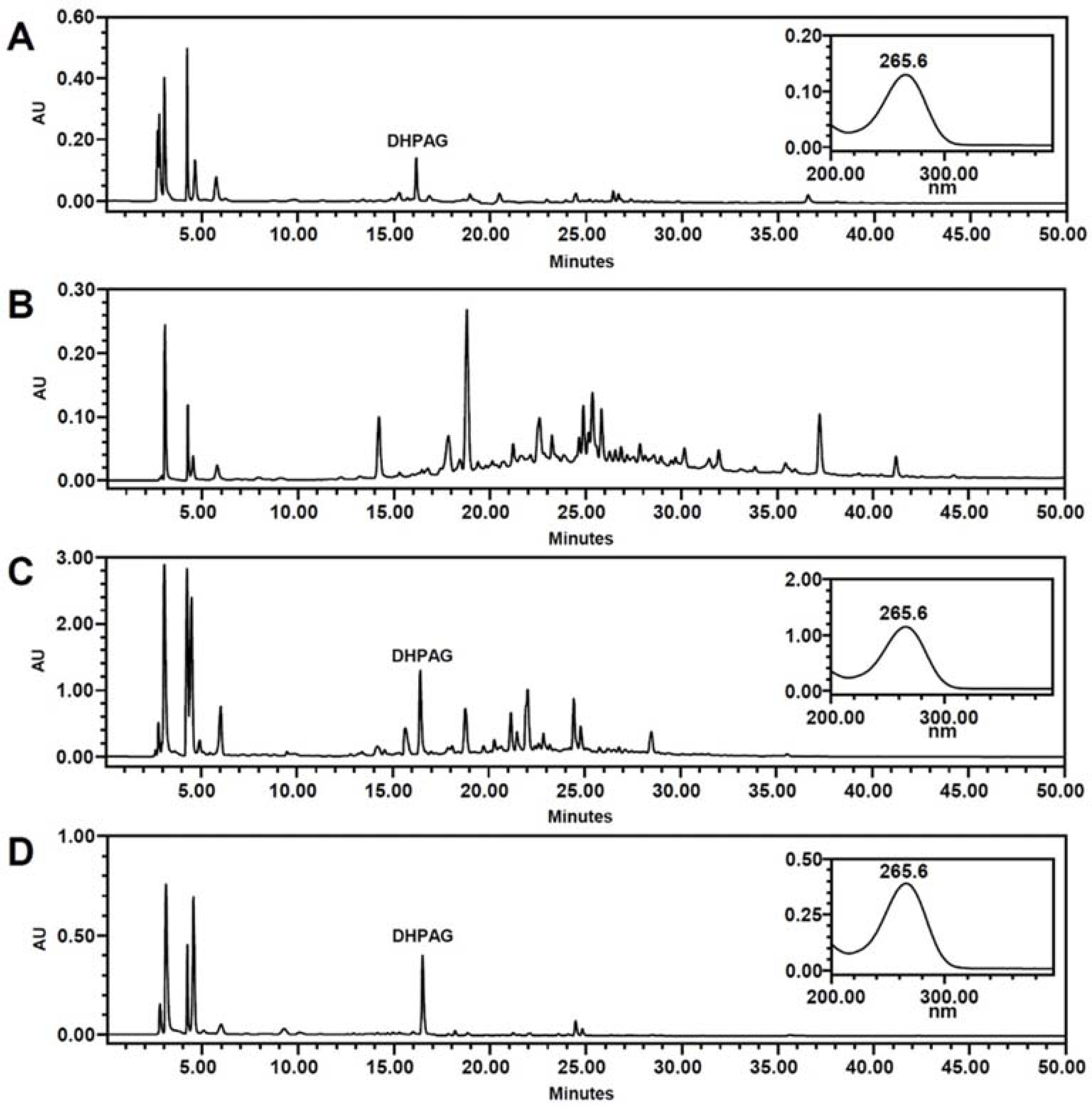
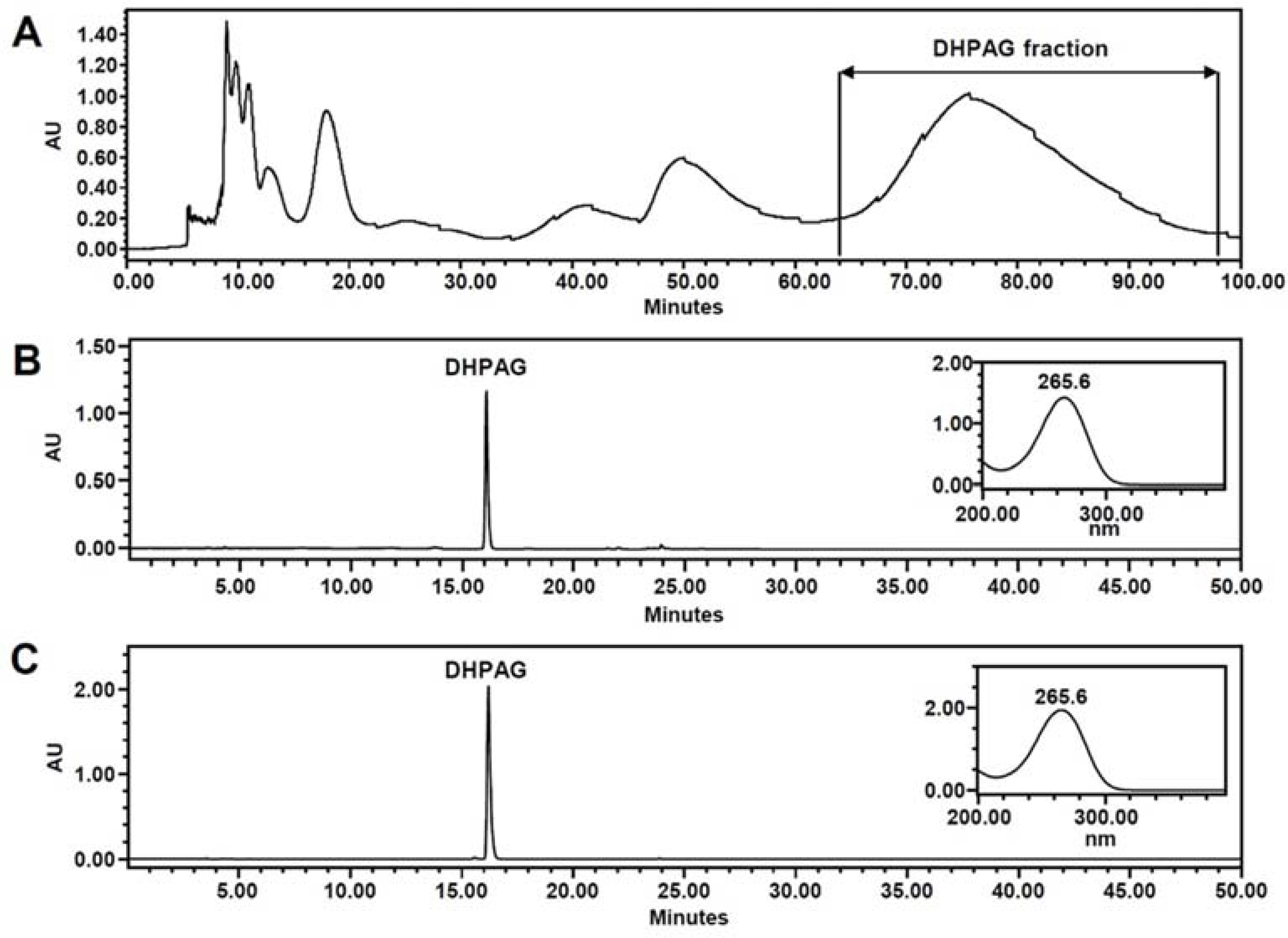
- High-performance countercurrent chromatography (HPCCC) coupled with reversed-phase high-performance liquid chromatography (RP-HPLC) method was developed to isolate dihydrophaseic acid 3"²-O-β-D-glucopyranoside (DHPAG) from the extract of Nelumbo nucifera seeds. Enriched DHPAG sample (2.3 g) was separated by HPCCC using ethyl acetate/n-butanol/water system (6:4:10, v/v/v, normal-phase mode, flow rate: 4.0 mL/min) to give 23.1 mg of DHPAG with purity of 88.7%. Further preparative RP-HPLC experiment gave pure DHPAG (16.3 mg, purity > 98%). The current study demonstrates that utilization of CCC method maximizes the isolation efficiency compared with that of solid-based conventional column chromatography.
Keyword
Figure
Reference
-
(1). Paudel K. R., Panth N.Evid. Based Complement. Alternat. Med. 2015; 789124.(2). Pharmacognosy. The Compilation Committee of Pharmacognosy Textbook. Dong-Myeung press;Korea: 2015. p. 358–360.(3). Rai S., Wahile A., Mukherjee K., Saha B. P., Mukherjee P. K. J.Ethnopharmacol. 2006; 104:322–327.(4). Lee H. K., Choi Y. M., Noh D. O., Suh H. J.Int. J. Food Sci. Technol. 2005; 40:709–715.(5). Yen G. C., Duh P. D., Su H. J., Yeh C. T., Wu C. H.Food Chem. 2006; 94:596–602.(6). Ling Z. Q., Xie B. J., Yang E. L. J.Agric. Food Chem. 2005; 53:2441–2445.(7). Duan Y., Zhang H., Xie B., Yan Y., Li J., Xu F., Qin Y.Food Chem. Toxicol. 2010; 48:3374–3384.(8). Kumaran A., Ho C. C., Hwang L. S. J.Food Drug Anal. 2018; 26:172–181.(9). Jung H. A., Jung Y. J., Hyun S. K., Min B. S., Kim D. W., Jung J. H, Choi J. S.Biol. Pharm. Bull. 2010; 33:267–272.(11). Rho T., Yoon K. D.Nat. Prod. Sci. 2017; 23:253–257.(12). Seo J. H., Choi Y. H., Yoo M. Y., Hong K. S., Lee B. H., Yon G. H., Kim Y. S., Kim Y. K., Ryu S. Y. Kor. J.Pharmacogn. 2006; 37:290–293.(13). Joung U., Lee J., Nam J. W., Lee Y. J., Seo E. K.Bull. Korean Chem. Soc. 2011; 32:4083–4085.(14). Park E., Kim M. C., Choi C. W., Kim J., Jin H. S., Lee R., Lee J. W., Park J. H., Huh D., Jeong S. Y.Molecules. 2016; 21:1260.(15). Ito Y. J.Chromatogr. A. 2005; 1065:145–168.(16). Fang Y., Li Q., Shao Q., Wang B., Wei Y. J.Chromatogr. A. 2017; 1507:63–71.(17). Wu L., Xiong W., Hu J. W., Gu Z., Xu J. G., Si C. L., Bae Y. S., Xu G. J.Chromatogr. Sci. 2018; 56:108–114.(18). Xingfeng G., Daijie W., Wenjuan D., Jinhua D., Xiao W.Phytochem. Anal. 2010; 21:268–272.
Article(19). del Refugio Ramos M., Jerz G., Villanueva S., López-Dellamary F., Waibel R., Winterhalter P.Phytochemistry. 2004; 65:955–962.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemical Constituents of Nelumbo nucifera Seeds
- Isolation And Development Of Quantification Method For Cyanidin-3-glucoside And Cyanidin-3-rutinoside In Mulberry Fruit By High-performance Countercurrent Chromatography And High-performance Liquid Chromatography
- Rapid Isolation of Cyanidin 3-Glucoside and Peonidin 3-Glucoside from Black Rice (Oryza sativa) Using High-Performance Countercurrent Chromatography and Reversed-Phase Column Chromatography
- Development of high-performance liquid chromatography methods for the anticoccidials: toltrazuril and diclazuril
- Bioequivalence test of two ciprofloxacin tablet preparations using high performance liquid chromatography