Nat Prod Sci.
2018 Dec;24(4):225-228. 10.20307/nps.2018.24.4.225.
Gliotoxin is Antibacterial to Drug-resistant Piscine Pathogens
- Affiliations
-
- 1College of Pharmacy, Pusan National University, Busan 609-735, Republic of Korea. jhjung@pusan.ac.kr
- 2College of Pharmacy, Kyung Hee University, Seoul 130-701, Republic of Korea.
- KMID: 2432415
- DOI: http://doi.org/10.20307/nps.2018.24.4.225
Abstract
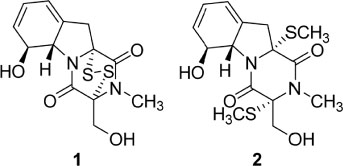
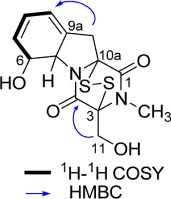
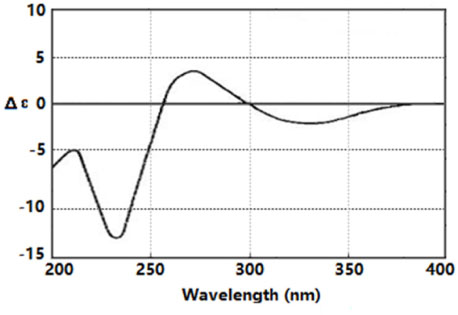
- By activity-guided fractionation, gliotoxin was isolated as an antibacterial metabolite of the fungus Penicillium decumbens which was derived from the jellyfish Nemopilema nomurai. Gliotoxin was further evaluated for antibacterial activity against several piscine and human MDR (multidrug resistance) pathogens. Gliotoxin showed significant antibacterial activity against Gram-positive piscine pathogens such as Streptococcus iniae FP5228, Streptococcus iniae FP3187, Streptococcus parauberis FP3287, Streptococcus parauberis SPOF3K, S. parauberis KSP28, and Lactococcus garvieae FP5245. Gliotoxin showed strong activity especially against S. parauberis SPOF3K and S. iniae FP5228, which are resistant to oxytetracycline. It is noteworthy that gliotoxin effectively suppressed streptococci which are the major pathogens for piscine infection and mortality in aquaculture industry. Gliotoxin also showed strong antibacterial activity against multidrug-resistant human pathogens (MDR) including Enterococcus faecium 5270 and MRSA (methicillin-resistant Staphylococcus aureus) 3089.
Keyword
MeSH Terms
Figure
Reference
-
1. Zhang HW, Song YC, Tan RX. Nat Prod Rep. 2006; 23:753–771.2. Bugni TS, Ireland CM. Nat Prod Rep. 2004; 21:143–163.3. Zhang Y, Mu J, Feng Y, Kang Y, Zhang J, Gu PJ, Wang Y, Ma LE, Zhu YH. Mar Drugs. 2009; 7:97–112.4. Ingavat N, Dobereiner J, Wiyakrutta S, Mahidol C, Ruchirawat S, Kittakoop P. J Nat Prod. 2009; 72:2049–2052.5. Jee BY, Shin KW, Lee DW, Kim YJ, Lee MK. J Fish Pathol. 2014; 27:77–83.6. Aoki T, Arai T, Egusa S. Microbiol Immunol. 1977; 21:77–83.7. Johnson John R, Bruce William F, Dutcher James D. J Am Chem Soc. 1943; 65:2005–2009.8. Nagarajan R, Woody RW. J Am Chem Soc. 1973; 95:7212–7222.9. Li X, Kim SK, Nam KW, Kang JS, Choi HD, Son BW. J Antibiot (Tokyo). 2006; 59:248–250.10. Vigushin DM, Mirsaidi N, Brooke G, Sun C, Pace P, Inman L, Moody CJ, Coombes RC. Med Oncol. 2004; 21:21–30.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Strategies to combat Gram-negative bacterial resistance to conventional antibacterial drugs: a review
- In vitro Activities of Oral Cephem and Telithromycin Against Clinical Isolates of Major Respiratory Pathogens in Japan
- The Effect of Protein Kinase C Pretreatment on Gliotoxin Induced Apoptosis in H9c2 Cells
- Gliotoxin induces the Apoptosis in HL-60 Cells
- Antimicrobial Therapy for Infections Caused by Multidrug-Resistant Gram-Negative Bacteria