Allergy Asthma Immunol Res.
2019 Mar;11(2):212-221. 10.4168/aair.2019.11.2.212.
Phenotypes of Severe Cutaneous Adverse Reactions Caused by Nonsteroidal Anti-inflammatory Drugs
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.
- 2Institute of Allergy and Clinical Immunology, Seoul National University Medical Research Center, Seoul, Korea.
- 3Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea.
- 4Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea.
- 5Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 6Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea.
- 7Department of Internal Medicine, Ewha Womans University College of Medicine, Seoul, Korea.
- 8Department of Internal Medicine, Dongguk University Ilsan Hospital, Goyang, Korea.
- 9Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 10Department of Internal Medicine, Pusan National University College of Medicine, Busan, Korea.
- 11Department of Pharmacology, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 12Department of Internal Medicine, Inje University Busan Paik Hospital, Busan, Korea.
- 13Department of Internal Medicine, Chonbuk National University Medical School, Jeonju, Korea.
- 14Department of Internal Medicine, Chosun University Hospital, Gwangju, Korea.
- 15Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea.
- 16Department of Internal Medicine, Keimyung University Dongsan Medical Center, Daegu, Korea.
- 17Department of Internal Medicine, Inha University School of Medicine, Incheon, Korea.
- 18Department of Internal Medicine, SMG-SNU Boramae Medical Center, Seoul, Korea.
- 19Department of Internal Medicine, Chungbuk National University Hospital, Cheongju, Korea.
- 20Department of Dermatology, Kangdong Sacred Heart Hospital, Seoul, Korea.
- 21Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea.
- 22Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea.
- 23Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea.
- 24Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea.
- 25Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea.
- 26Department of Internal Medicine, Inje University Haeundae Paik Hospital, Busan, Korea.
- 27Department of Internal Medicine, Gyeongsang National University School of Medicine, Jinju, Korea.
- 28Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. ye9007@ajou.ac.kr
- KMID: 2431853
- DOI: http://doi.org/10.4168/aair.2019.11.2.212
Abstract
- PURPOSE
Nonsteroidal anti-inflammatory drugs (NSAIDs) are common cause of severe cutaneous adverse reactions (SCARs). The present study aimed to investigate the characteristics of SCARs induced by NSAIDs in the Korean SCAR registry.
METHODS
A retrospective survey of NSAID-induced SCARs recorded between 2010 and 2015 at 27 university hospitals in Korea was conducted. Clinical phenotypes of SCARs were classified into Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), SJS-TEN overlap syndrome and drug reaction with eosinophilia and systemic symptoms (DRESS). Causative NSAIDs were classified into 7 groups according to their chemical properties: acetaminophen, and propionic, acetic, salicylic, fenamic and enolic acids.
RESULTS
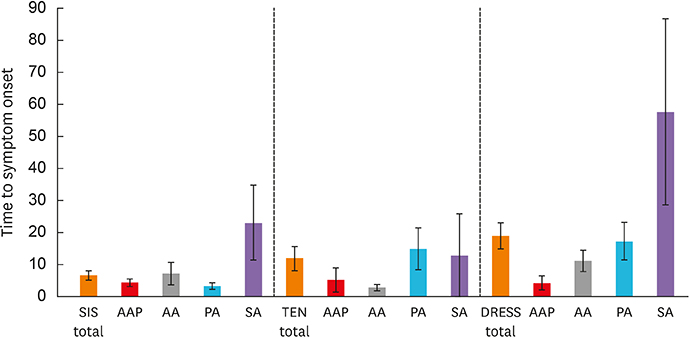
A total of 170 SCARs, consisting of 85 SJS, 32 TEN, 17 SJS-TEN overlap syndrome and 36 DRESS reactions, were induced by NSAIDs: propionic acids (n=68), acetaminophen (n=38), acetic acids (n=23), salicylic acids (n=16), coxibs (n=8), fenamic acids (n=7), enolic acids (n=5) and unclassified (n=5). Acetic acids (22%) and coxibs (14%) accounted for higher portions of DRESS than other SCARs. The phenotypes of SCARs induced by both propionic and salicylic acids were similar (SJS, TEN and DRESS, in order). Acetaminophen was primarily associated with SJS (27%) and was less involved in TEN (10%). DRESS occurred more readily among subjects experiencing coxib-induced SCARs than other NSAID-induced SCARs (62.5% vs. 19.7%, P = 0.013). The mean time to symptom onset was longer in DRESS than in SJS or TEN (19.1 ± 4.1 vs. 6.8 ±1.5 vs. 12.1 ± 3.8 days). SCARs caused by propionic salicylic acids showed longer latency, whereas acetaminophen- and acetic acid-induced SCARs appeared within shorter intervals.
CONCLUSIONS
The present study indicates that the phenotypes of SCARs may differ according to the chemical classifications of NSAIDs. To establish the mechanisms and incidences of NSAID-induced SCARs, further prospective studies are needed.
MeSH Terms
-
Acetaminophen
Acetates
Acetic Acid
Anti-Inflammatory Agents, Non-Steroidal
Cicatrix
Classification
Cyclooxygenase 2 Inhibitors
Diethylpropion
Drug Hypersensitivity
Drug Hypersensitivity Syndrome
Hospitals, University
Incidence
Korea
Phenotype*
Propionates
Prospective Studies
Retrospective Studies
Salicylates
Salicylic Acid
Stevens-Johnson Syndrome
Acetaminophen
Acetates
Acetic Acid
Anti-Inflammatory Agents, Non-Steroidal
Cyclooxygenase 2 Inhibitors
Diethylpropion
Propionates
Salicylates
Salicylic Acid
Figure
Cited by 1 articles
-
Update on the Management of Nonsteroidal Anti-Inflammatory Drug Hypersensitivity
Wan Yin Winnie Yeung, Hae Sim Park
Yonsei Med J. 2020;61(1):4-14. doi: 10.3349/ymj.2020.61.1.4.
Reference
-
1. Ajayi FO, Sun H, Perry J. Adverse drug reactions: a review of relevant factors. J Clin Pharmacol. 2000; 40:1093–1101.2. Naldi L, Conforti A, Venegoni M, Troncon MG, Caputi A, Ghiotto E, et al. Cutaneous reactions to drugs. An analysis of spontaneous reports in four Italian regions. Br J Clin Pharmacol. 1999; 48:839–846.
Article3. Finkelstein Y, Macdonald EM, Li P, Hutson JR, Juurlink DN. Recurrence and mortality following severe cutaneous adverse reactions. JAMA. 2014; 311:2231–2232.
Article4. Paulmann M, Mockenhaupt M. Severe drug-induced skin reactions: clinical features, diagnosis, etiology, and therapy. J Dtsch Dermatol Ges. 2015; 13:625–645.
Article5. Kidon MI, See Y. Adverse drug reactions in Singaporean children. Singapore Med J. 2004; 45:574–577.6. Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004; 329:15–19.
Article7. Mastalerz L, Setkowicz M, Szczeklik A. Mechanism of chronic urticaria exacerbation by aspirin. Curr Allergy Asthma Rep. 2005; 5:277–283.
Article8. Szczeklik A, Sanak M. The broken balance in aspirin hypersensitivity. Eur J Pharmacol. 2006; 533:145–155.
Article9. Canto MG, Andreu I, Fernandez J, Blanca M. Selective immediate hypersensitivity reactions to NSAIDs. Curr Opin Allergy Clin Immunol. 2009; 9:293–297.
Article10. Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995; 333:1600–1607.
Article11. Levi N, Bastuji-Garin S, Mockenhaupt M, Roujeau JC, Flahault A, Kelly JP, et al. Medications as risk factors of Stevens-Johnson syndrome and toxic epidermal necrolysis in children: a pooled analysis. Pediatrics. 2009; 123:e297–e304.
Article12. Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007; 156:609–611.
Article13. Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993; 129:92–96.
Article14. Meyboom RH, Hekster YA, Egberts AC, Gribnau FW, Edwards IR. Causal or casual? the role of causality assessment in pharmacovigilance. Drug Saf. 1997; 17:374–389.15. Duong TA, Valeyrie-Allanore L, Wolkenstein P, Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet. 2017; 390:1996–2011.
Article16. Kowalski ML, Makowska JS, Blanca M, Bavbek S, Bochenek G, Bousquet J, et al. Hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) - classification, diagnosis and management: review of the EAACI/ENDA(#) and GA2LEN/HANNA*. Allergy. 2011; 66:818–829.
Article17. Mockenhaupt M, Kelly JP, Kaufman D, Stern RS. SCAR Study Group. The risk of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with nonsteroidal antiinflammatory drugs: a multinational perspective. J Rheumatol. 2003; 30:2234–2240.18. Doña I, Blanca-López N, Cornejo-García JA, Torres MJ, Laguna JJ, Fernández J, et al. Characteristics of subjects experiencing hypersensitivity to non-steroidal anti-inflammatory drugs: patterns of response. Clin Exp Allergy. 2011; 41:86–95.
Article19. Díaz Jara M, Pérez Montero A, Gracia Bara MT, Cabrerizo S, Zapatero L, Martínez Molero MI. Allergic reactions due to ibuprofen in children. Pediatr Dermatol. 2001; 18:66–67.20. Kowalski ML, Asero R, Bavbek S, Blanca M, Blanca-Lopez N, Bochenek G, et al. Classification and practical approach to the diagnosis and management of hypersensitivity to nonsteroidal anti-inflammatory drugs. Allergy. 2013; 68:1219–1232.
Article21. Cornejo-Garcia JA, Blanca-López N, Doña I, Andreu I, Agúndez JA, Carballo M, et al. Hypersensitivity reactions to non-steroidal anti-inflammatory drugs. Curr Drug Metab. 2009; 10:971–980.
Article22. Blanca-López N, Bogas G, Doña I, Torres MJ, Blanca M, Cornejo-García JA, et al. ASA must be given to classify multiple NSAID-hypersensitivity patients as selective or cross-intolerant. Allergy. 2016; 71:576–578.
Article23. Demir S, Olgac M, Unal D, Gelincik A, Colakoglu B, Buyukozturk S. Evaluation of hypersensitivity reactions to nonsteroidal anti-inflammatory drugs according to the latest classification. Allergy. 2015; 70:1461–1467.
Article24. La Grenade L, Lee L, Weaver J, Bonnel R, Karwoski C, Governale L, et al. Comparison of reporting of Stevens-Johnson syndrome and toxic epidermal necrolysis in association with selective COX-2 inhibitors. Drug Saf. 2005; 28:917–924.
Article25. Iñiguez MA, Punzón C, Fresno M. Induction of cyclooxygenase-2 on activated T lymphocytes: regulation of T cell activation by cyclooxygenase-2 inhibitors. J Immunol. 1999; 163:111–119.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The frequency of adverse drug reactions in a tertiary care hostpital in Korea
- Seven Steps to the Diagnosis of NSAIDs Hypersensitivity: How to Apply a New Classification in Real Practice?
- Hypersensitivity to Aspirin and Nonsteroidal Anti-inflammatory Drugs
- Social Burden of Drug Allergy and its Prevention
- Cutaneous adverse drug reactions