Yonsei Med J.
2019 Feb;60(2):163-173. 10.3349/ymj.2019.60.2.163.
MicroRNA-206 Reduces Osteosarcoma Cell Malignancy In Vitro by Targeting the PAX3-MET Axis
- Affiliations
-
- 1Department of Spine Surgery, Chongqing Three Gorges Central Hospital, Chongqing, China.
- 2Department of Neurology, Chongqing Three Gorges Central Hospital, Chongqing, China.
- 3Department of Internal Medicine, Chongqing Wanzhou District Traditional Chinese Hospital, Chongqing, China. dengqr2008@sina.com
- KMID: 2431633
- DOI: http://doi.org/10.3349/ymj.2019.60.2.163
Abstract
- PURPOSE
This study was undertaken to explore how miR-206 represses osteosarcoma (OS) development.
MATERIALS AND METHODS
Expression levels of miR-206, PAX3, and MET mRNA were explored in paired OS and adjacent tissue specimens. A patient-derived OS cell line was established. miR-206 overexpression and knockdown were achieved by lentiviral transduction. PAX3 and MET overexpression were achieved by plasmid transfection. Treatment with hepatocyte growth factor (HGF) was utilized to activate c-Met receptor. Associations between miR-206 and PAX3 or MET mRNA in OS cells were verified by AGO2-RNA immunoprecipitation assay and miRNA pulldown assay. OS cell malignancy was evaluated in vitro by cell proliferation, metastasis, and apoptosis assays. PAX3 and MET gene expression in OS cells was assayed by RT-qPCR and Western blot. Activation of PI3K-AKT and MAPK-ERK in OS cells were assayed by evaluating Akt1 Ser473 phosphorylation and total threonine phosphorylation of Erk1/2, respectively.
RESULTS
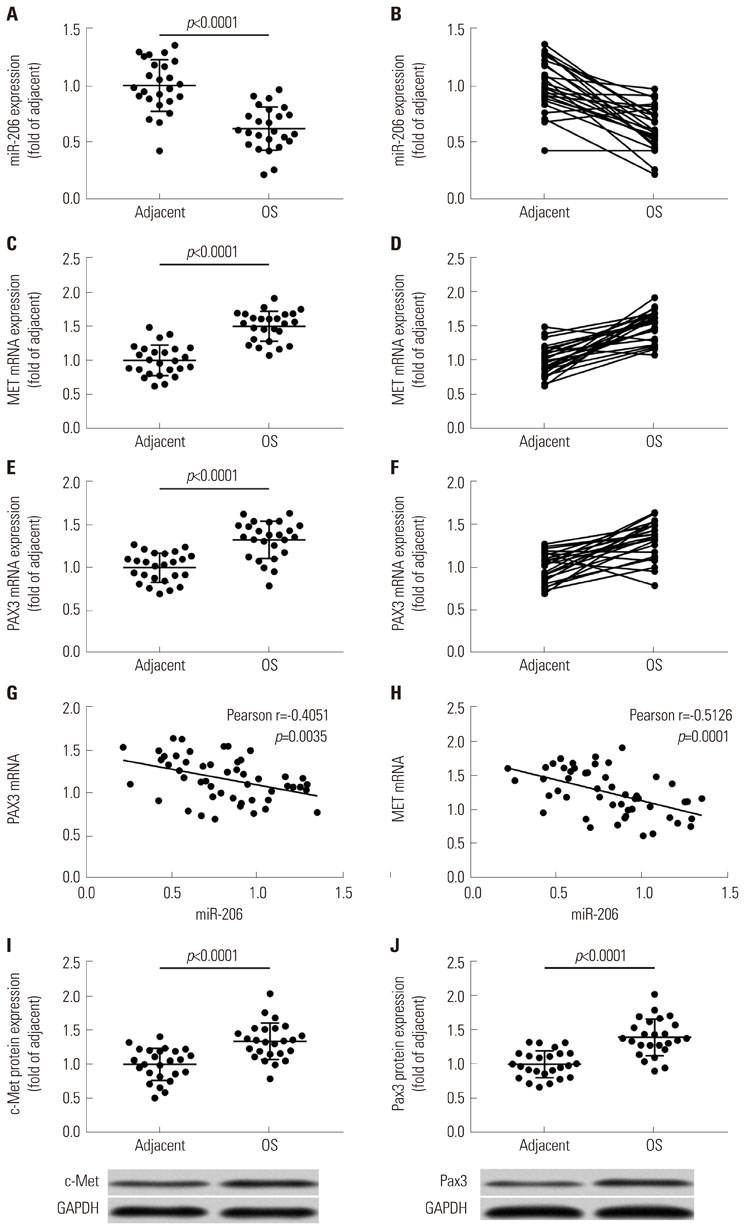
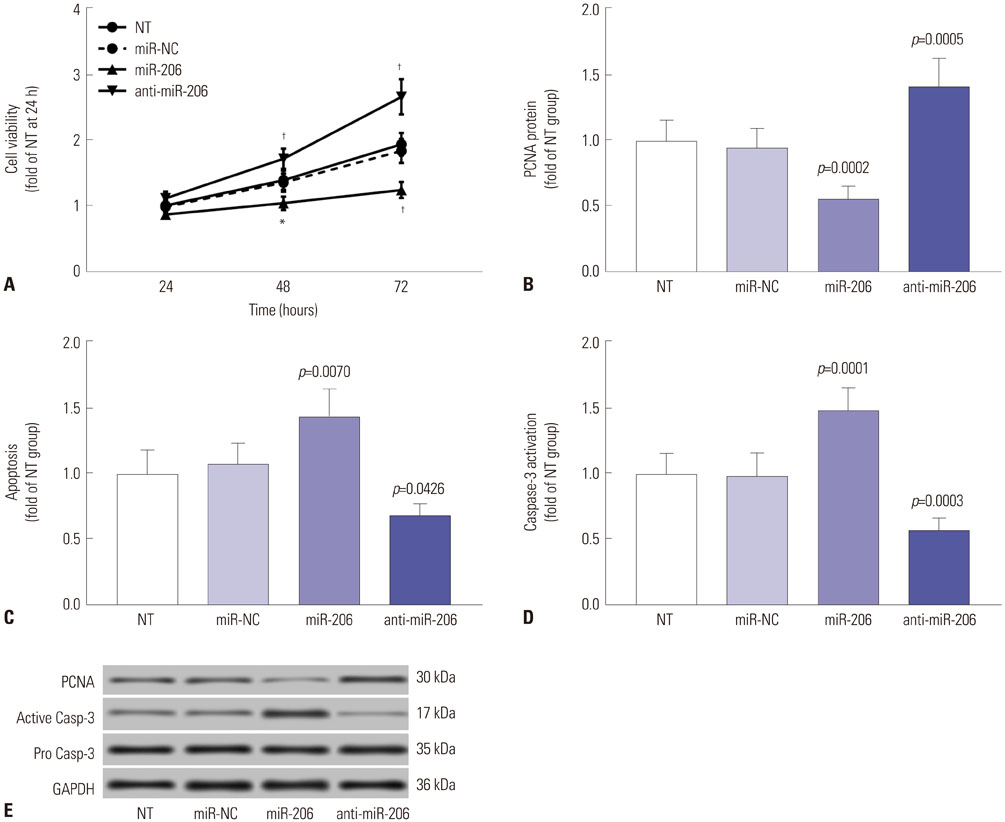
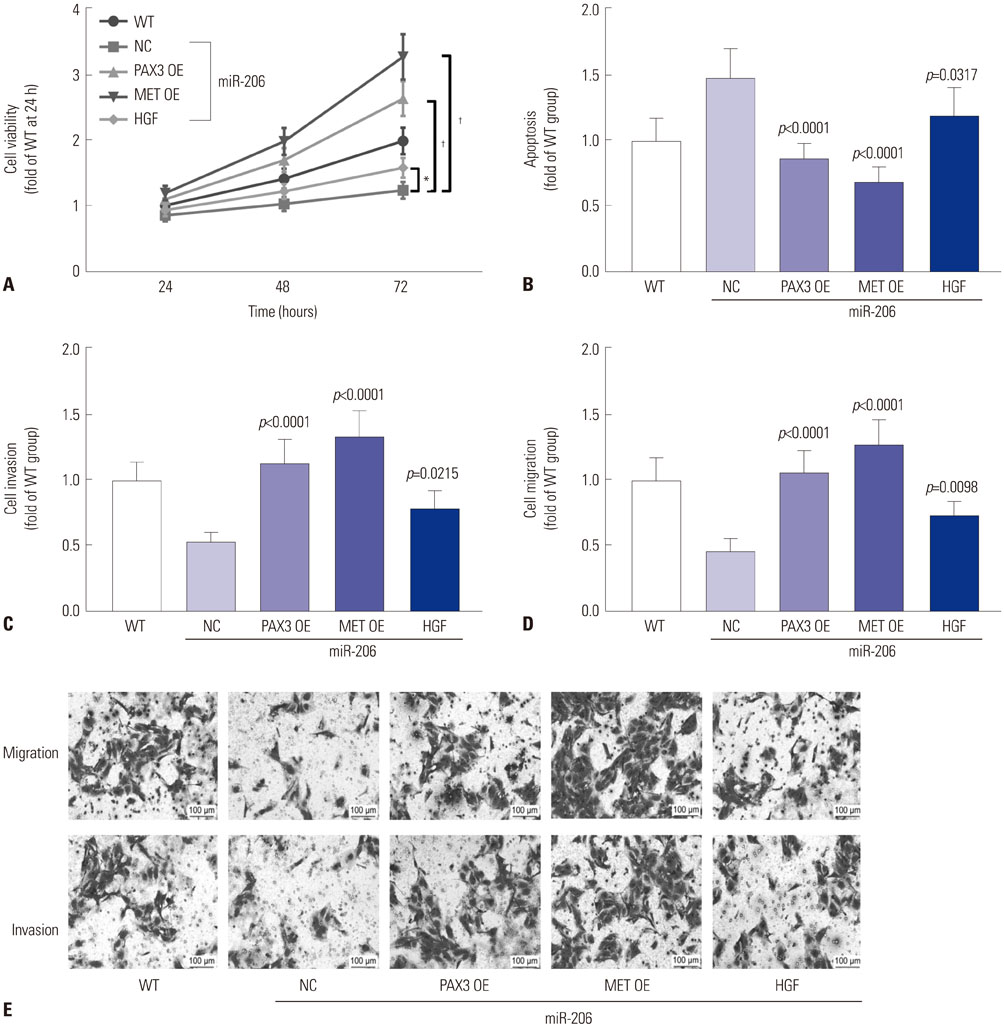
Expression levels of miR-206 were significantly decreased in OS tissue specimens, compared to adjacent counterparts, and were inversely correlated with expression of PAX3 and MET mRNA. miR-206 directly interacted with PAX3 and MET mRNA in OS cells. miR-206 overexpression significantly reduced PAX3 and MET gene expression in OS cells in vitro, resulting in significant decreases in Akt1 and Erk1/2 activation, cell proliferation, and metastasis, as well as increases in cell apoptosis, while miR-206 knockdown showed the opposite effects. The effects of miR-206 overexpression on OS cells were reversed by PAX3 or MET overexpression, but only partially attenuated by HGF treatment.
CONCLUSION
miR-206 reduces OS cell malignancy in vitro by targeting PAX3 and MET gene expression.
MeSH Terms
Figure
Reference
-
1. Gianferante DM, Mirabello L, Savage SA. Germline and somatic genetics of osteosarcoma - connecting aetiology, biology and therapy. Nat Rev Endocrinol. 2017; 13:480–491.
Article2. Gill J, Ahluwalia MK, Geller D, Gorlick R. New targets and approaches in osteosarcoma. Pharmacol Ther. 2013; 137:89–99.
Article3. Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer. 2014; 14:722–735.
Article4. Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010; 21:Suppl 7. vii320–vii325.
Article5. Iwakawa HO, Tomari Y. The functions of microRNAs: mRNA decay and translational repression. Trends Cell Biol. 2015; 25:651–665.
Article6. Ram Kumar RM, Boro A, Fuchs B. Involvement and clinical aspects of microRNA in osteosarcoma. Int J Mol Sci. 2016; 17:E877.
Article7. Kim YH, Goh TS, Lee CS, Oh SO, Kim JI, Jeung SH, et al. Prognostic value of microRNAs in osteosarcoma: a meta-analysis. Oncotarget. 2017; 8:8726–8737.
Article8. Palmini G, Marini F, Brandi ML. What is new in the miRNA world regarding osteosarcoma and chondrosarcoma? Molecules. 2017; 22:E417.
Article9. Namløs HM, Meza-Zepeda LA, Barøy T, Østensen IH, Kresse SH, Kuijjer ML, et al. Modulation of the osteosarcoma expression phenotype by microRNAs. PLoS One. 2012; 7:e48086.
Article10. Bao YP, Yi Y, Peng LL, Fang J, Liu KB, Li WZ, et al. Roles of microRNA-206 in osteosarcoma pathogenesis and progression. Asian Pac J Cancer Prev. 2013; 14:3751–3755.
Article11. Zhang C, Yao C, Li H, Wang G, He X. Serum levels of microRNA-133b and microRNA-206 expression predict prognosis in patients with osteosarcoma. Int J Clin Exp Pathol. 2014; 7:4194–4203.12. Pan BL, Tong ZW, Wu L, Pan L, Li JE, Huang YG, et al. Effects of microRNA-206 on osteosarcoma cell proliferation, apoptosis, migration and invasion by targeting ANXA2 through the AKT signaling pathway. Cell Physiol Biochem. 2018; 45:1410–1422.
Article13. Di Leva G, Gasparini P, Piovan C, Ngankeu A, Garofalo M, Taccioli C, et al. MicroRNA cluster 221-222 and estrogen receptor alpha interactions in breast cancer. J Natl Cancer Inst. 2010; 102:706–721.
Article14. Zhang L, Xia L, Zhao L, Chen Z, Shang X, Xin J, et al. Activation of PAX3-MET pathways due to miR-206 loss promotes gastric cancer metastasis. Carcinogenesis. 2015; 36:390–399.
Article15. Yan D, Dong Xda E, Chen X, Wang L, Lu C, Wang J, et al. MicroRNA-1/206 targets c-Met and inhibits rhabdomyosarcoma development. J Biol Chem. 2009; 284:29596–29604.
Article16. Hirai H, Verma M, Watanabe S, Tastad C, Asakura Y, Asakura A. MyoD regulates apoptosis of myoblasts through microRNA-mediated down-regulation of Pax3. J Cell Biol. 2010; 191:347–365.
Article17. Liu Q, Yang G, Qian Y. Loss of MicroRNA-489-3p promotes osteosarcoma metastasis by activating PAX3-MET pathway. Mol Carcinog. 2017; 56:1312–1321.
Article18. Rees H, Williamson D, Papanastasiou A, Jina N, Nabarro S, Shipley J, et al. The MET receptor tyrosine kinase contributes to invasive tumour growth in rhabdomyosarcomas. Growth Factors. 2006; 24:197–208.
Article19. Patané S, Avnet S, Coltella N, Costa B, Sponza S, Olivero M, et al. MET overexpression turns human primary osteoblasts into osteosarcomas. Cancer Res. 2006; 66:4750–4757.
Article20. Dani N, Olivero M, Mareschi K, van Duist MM, Miretti S, Cuvertino S, et al. The MET oncogene transforms human primary bone-derived cells into osteosarcomas by targeting committed osteoprogenitors. J Bone Miner Res. 2012; 27:1322–1334.
Article21. Husmann K, Ducommun P, Sabile AA, Pedersen EM, Born W, Fuchs B. Signal transduction and downregulation of C-MET in HGF stimulated low and highly metastatic human osteosarcoma cells. Biochem Biophys Res Commun. 2015; 464:1222–1227.
Article22. Li X, Sun X, Wu J, Li Z. MicroRNA-613 suppresses proliferation, migration and invasion of osteosarcoma by targeting c-MET. Am J Cancer Res. 2016; 6:2869–2879.23. Niu G, Li B, Sun J, Sun L. miR-454 is down-regulated in osteosarcomas and suppresses cell proliferation and invasion by directly targeting c-Met. Cell Prolif. 2015; 48:348–355.
Article24. Zhao H, Li M, Li L, Yang X, Lan G, Zhang Y. MiR-133b is down-regulated in human osteosarcoma and inhibits osteosarcoma cells proliferation, migration and invasion, and promotes apoptosis. PLoS One. 2013; 8:e83571.
Article25. Yan K, Gao J, Yang T, Ma Q, Qiu X, Fan Q, et al. MicroRNA-34a inhibits the proliferation and metastasis of osteosarcoma cells both in vitro and in vivo. PLoS One. 2012; 7:e33778.
Article26. Blattmann C, Thiemann M, Stenzinger A, Roth EK, Dittmar A, Witt H, et al. Establishment of a patient-derived orthotopic osteosarcoma mouse model. J Transl Med. 2015; 13:136.
Article27. Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. 2015; 33:3029–3035.
Article28. Scagliotti GV, Novello S, von Pawel J. The emerging role of MET/HGF inhibitors in oncology. Cancer Treat Rev. 2013; 39:793–801.
Article29. Kubic JD, Little EC, Lui JW, Iizuka T, Lang D. PAX3 and ETS1 synergistically activate MET expression in melanoma cells. Oncogene. 2015; 34:4964–4974.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute Myeloid Leukemia after Chemotherapy for Osteosarcoma: A Case Report
- miR-1301/TRIAP1 Axis Participates in Epirubicin-Mediated Anti-Proliferation and Pro-Apoptosis in Osteosarcoma
- Primary Osteosarcoma of the Breast: A case report
- Rosette-forming epithelioid osteosarcoma in the rib: a rare case of location and morphology
- Metachronous osteosarcoma