Ann Lab Med.
2019 May;39(3):322-326. 10.3343/alm.2019.39.3.322.
Age-Specific Cutoffs of the Sysmex UF-1000i Automated Urine Analyzer for Rapid Screening of Urinary Tract Infections in Outpatients
- Affiliations
-
- 1Department of Laboratory Medicine, Chung-Ang University Hospital, Chung-Ang University College of Medicine, Seoul, Korea. cpworld@cau.ac.kr
- 2Department of Urology, Chung-Ang University Hospital, Chung-Ang University College of Medicine, Seoul, Korea.
- KMID: 2431611
- DOI: http://doi.org/10.3343/alm.2019.39.3.322
Abstract
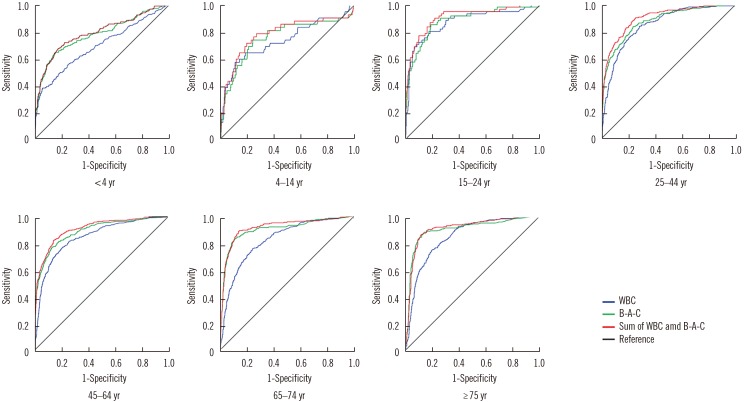
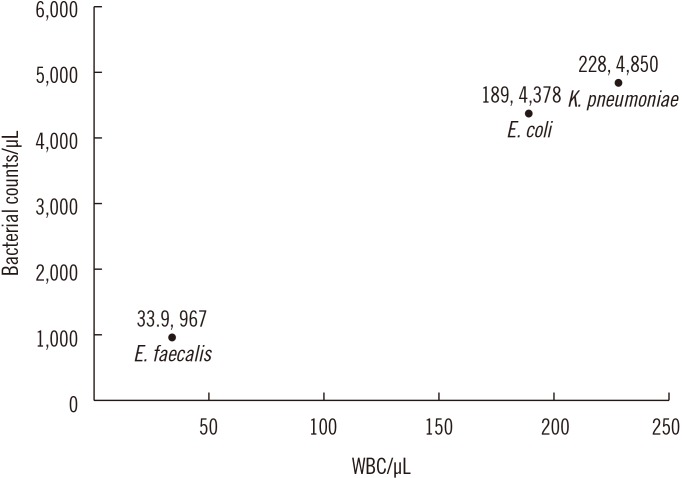
- We investigated the usefulness of age-specific cutoffs for screening of urinary tract infections (UTIs) in Korean outpatients, using the automated urine analyzer UF-1000i (Sysmex, Kobe, Japan). We retrospectively reviewed outpatient medical records. Urine samples of 7,443 outpatients from January 2010 to December 2017 were analyzed using urine culture and UF-1000i. ROC curves were calculated for each UF-1000i parameter based on the culture results. There were 1,398 culture positive samples, 5,774 culture negative samples, and 271 contaminated samples. UF-1000i had an area under the curve of ≥0.9 in outpatients >15 years. The appropriate cutoffs, which are the sum of bacterial (B-A-C) and white blood cell (WBC) counts, were 297.10/µL (15-24 years), 395.65/µL (25-44 years), 135.65/µL (45-64 years), 67.95/µL (65-74 years), and 96.5/µL (≥75 years). B-A-C and WBC counts differed among the three most frequently identified bacteria (Escherichia coli, Klebsiella pneumoniae, and Enterococcus faecalis). The UF-1000i system is useful for applying age-specific cutoffs to screen for UTIs, thereby preventing antibiotic abuse and reducing antibiotic resistance. Future studies can explore how its B-A-C and WBC counts can facilitate selection of empirical antibiotics by distinguishing between gram-positive and gram-negative bacteria.
MeSH Terms
Figure
Cited by 1 articles
-
Internal Quality Control Data of Urine Reagent Strip Tests and Derivation of Control Rules Based on Sigma Metrics
Haeil Park, Younsuk Ko
Ann Lab Med. 2021;41(5):447-454. doi: 10.3343/alm.2021.41.5.447.
Reference
-
1. Stamm WE, Norrby SR. Urinary tract infections: disease panorama and challenges. J Infect Dis. 2001; 183(S1):S1–S4. PMID: 11171002.2. Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. Vital Health Stat 13. 2011; 4. 1–38. PMID: 21614897.3. Schmiemann G, Kniehl E, Gebhardt K, Matejczyk MM, Hummers-Pradier E. The diagnosis of urinary tract infection: a systematic review. Dtsch Arztebl Int. 2010; 107:361–367. PMID: 20539810.4. Huh JS. The prevalence of urinary tract infections in institutionalized vs. noninstitutionalized elderly persons. Urogenit Tract Infect. 2016; 11:56–61.5. Lee KY. New insights for febrile urinary tract infection (acute pyelonephritis) in children. Child Kidney Dis. 2016; 20:37–44.6. Choe HS, Lee SJ, Cho YH, Çek M, Tandoğdu Z, Wagenlehner F, et al. Aspects of urinary tract infections and antimicrobial resistance in hospitalized urology patients in Asia: 10-year results of the Global Prevalence study of Infections in Urology (GPIU). J Infect Chemother. 2018; 24:278–283. PMID: 29292177.7. Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002; 287:2701–2710. PMID: 12020306.8. Kayalp D, Dogan K, Ceylan G, Senes M, Yucel D. Can routine automated urinalysis reduce culture requests? Clin Biochem. 2013; 46:1285–1289. PMID: 23810583.9. Oyaert M, Delanghe J. Progress in automated urinalysis. Ann Lab Med. 2019; 39:15–22. PMID: 30215225.10. Laiwejpithaya S, Wongkrajang P, Reesukumal K, Bucha C, Meepanya S, Pattanavin C, et al. UriSed 3 and UX-2000 automated urine sediment analyzers vs manual microscopic method: a comparative performance analysis. J Clin Lab Anal. 2018; 32:e22249.11. van der Zwet WC, Hessels J, Canbolat F, Deckers MM. Evaluation of the Sysmex UF-1000i® urine flow cytometer in the diagnostic work-up of suspected urinary tract infection in a Dutch general hospital. Clin Chem Lab Med. 2010; 48:1765–1771. PMID: 20726812.12. Hu X, Zhang J, Zhang X. Evaluation of the Sysmex UF-1000i urine analyzer as a screening test to reduce the need for urine cultures for urinary tract infection. Lab Med. 2010; 41:349–352.13. Íñigo M, Coello A, Fernández-Rivas G, Carrasco M, Marcó C, Fernández A, et al. Evaluation of the SediMax automated microscopy sediment analyzer and the Sysmex UF-1000i flow cytometer as screening tools to rule out negative urinary tract infections. Clin Chim Acta. 2016; 456:31–35. PMID: 26921459.14. Martín-Gutiérrez G, Porras-González A, Martín-Pérez C, Lepe JA, Aznar J. Evaluation and optimization of the Sysmex UF1000i system for the screening of urinary tract infection in primary health care elderly patients. Enferm Infecc Microbiol Clin. 2015; 33:320–323. PMID: 25444045.15. Pieretti B, Brunati P, Pini B, Colzani C, Congedo P, Rocchi M, et al. Diagnosis of bacteriuria and leukocyturia by automated flow cytometry compared with urine culture. J Clin Microbiol. 2010; 48:3990–3996. PMID: 20739491.16. Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management. Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011; 128:595–610. PMID: 21873693.17. LaRocco MT, Franek J, Leibach EK, Weissfeld AS, Kraft CS, Sautter RL, et al. Effectiveness of pre-analytic practices on contamination and diagnostic accuracy of urine cultures: a laboratory medicine best practices systematic review and meta-analysis. Clin Microbiol Rev. 2016; 29:105–147. PMID: 26598386.18. Monsen T, Rydín P. Flow cytometry analysis using Sysmex UF-1000i classifies uropathogens based on bacterial, leukocyte, and erythrocyte counts in urine specimens among patients with urinary tract infections. J Clin Microbiol. 2015; 53:539–545. PMID: 25472486.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of YD URiSCAN PluScope Urine Microscopic Analyzer and Sysmex UF-1000i Flow Cytometry Systems
- Selection of Unnecessary Urine Culture Specimens Using Sysmex UF-5000 Urine Flow Cytometer
- Performance of the Sysmex UF-1000i System in Screening for Significant Bacteriuria in Patients with Bladder Cancer Who Received Bacillus Calmette-Guérin Treatment
- Evaluation of an Automated Urine Flow Cytometer for Screening of Bacterial Contamination in Platelet Concentrates
- Small Red Blood Cell Fraction on the UF-1000i Urine Analyzer as a Screening Tool to Detect Dysmorphic Red Blood Cells for Diagnosing Glomerulonephritis