Ann Lab Med.
2019 May;39(3):237-244. 10.3343/alm.2019.39.3.237.
Accurate and Rapid Measurement of Glycated Hemoglobin Using HLC-723 G11 Variant Mode
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. nayadoo@hanmail.net
- 2Department of Laboratory Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- KMID: 2431600
- DOI: http://doi.org/10.3343/alm.2019.39.3.237
Abstract
- BACKGROUND
Type II diabetes mellitus causes many complications, and its prevalence continues to increase in Korea. Accurate measurement of glycated Hb (HbA1c) is important because of its usefulness in diagnosis, follow-up, and prediction of prognosis. We tested the analytical performance of the HLC-723 G11 Variant Mode (G11vr; Tosoh Bioscience, Inc., Tokyo, Japan), recently introduced to Korea, in detecting HbA1c.
METHODS
We evaluated precision, linearity, carry-over, and turnaround time. Using 208 samples, including 108 flagged samples, we compared HbA1c concentrations from four analyzers through correlation analysis: G11vr, HLC-723 G8 Variant Mode (G8vr, Tosoh Bioscience), HLC-723 G11 Standard Mode (G11st, Tosoh Bioscience), and HLC-723 G8 Standard Mode (G8st, Tosoh Bioscience). We used HPLC mass spectrometry (MS) and capillary electrophoresis (CE) to confirm the HbA1c concentrations of 15 additional known Hb variant samples.
RESULTS
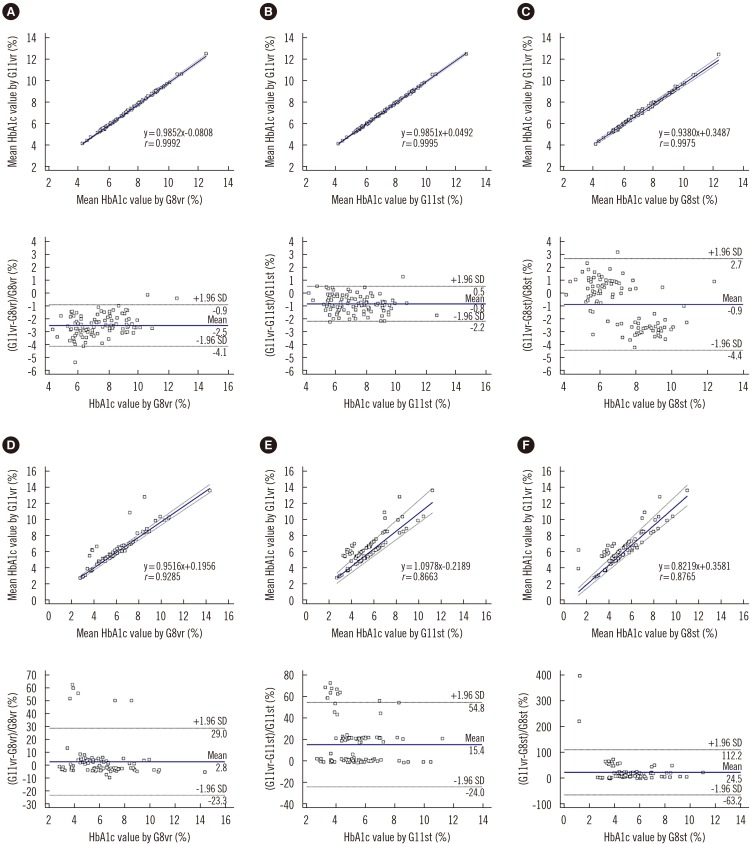
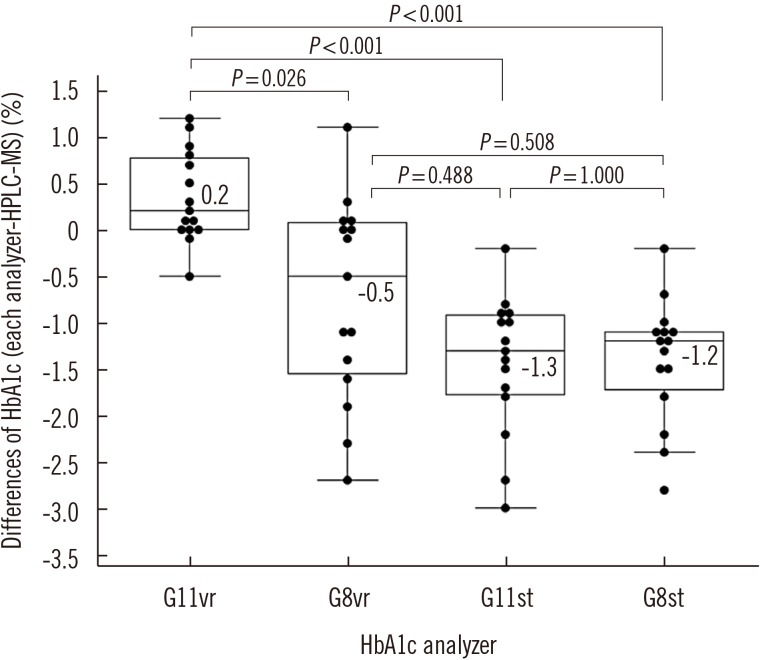
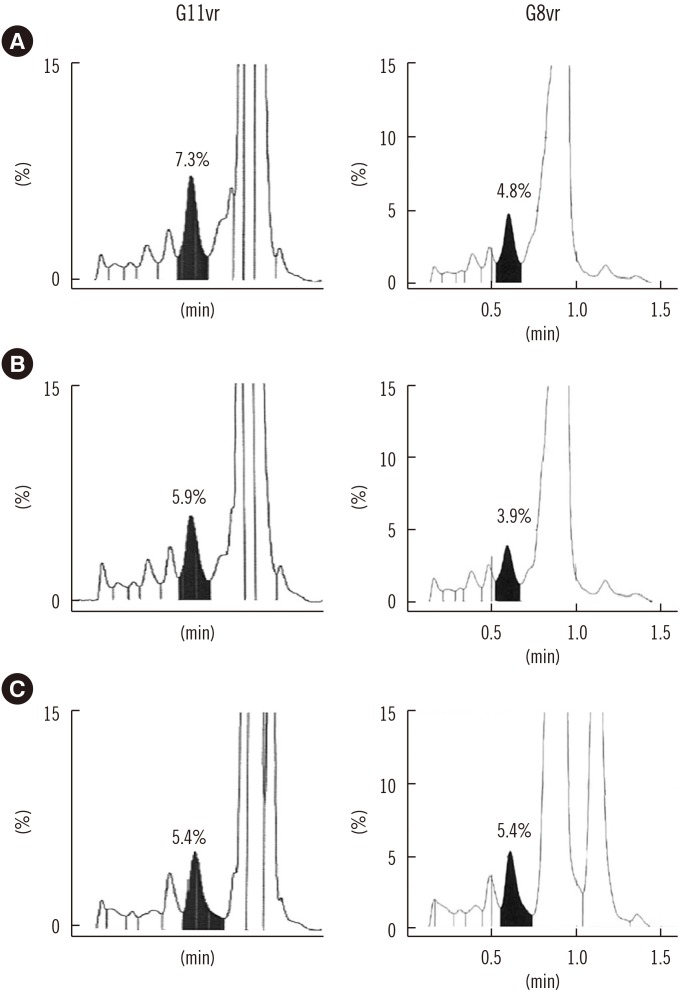
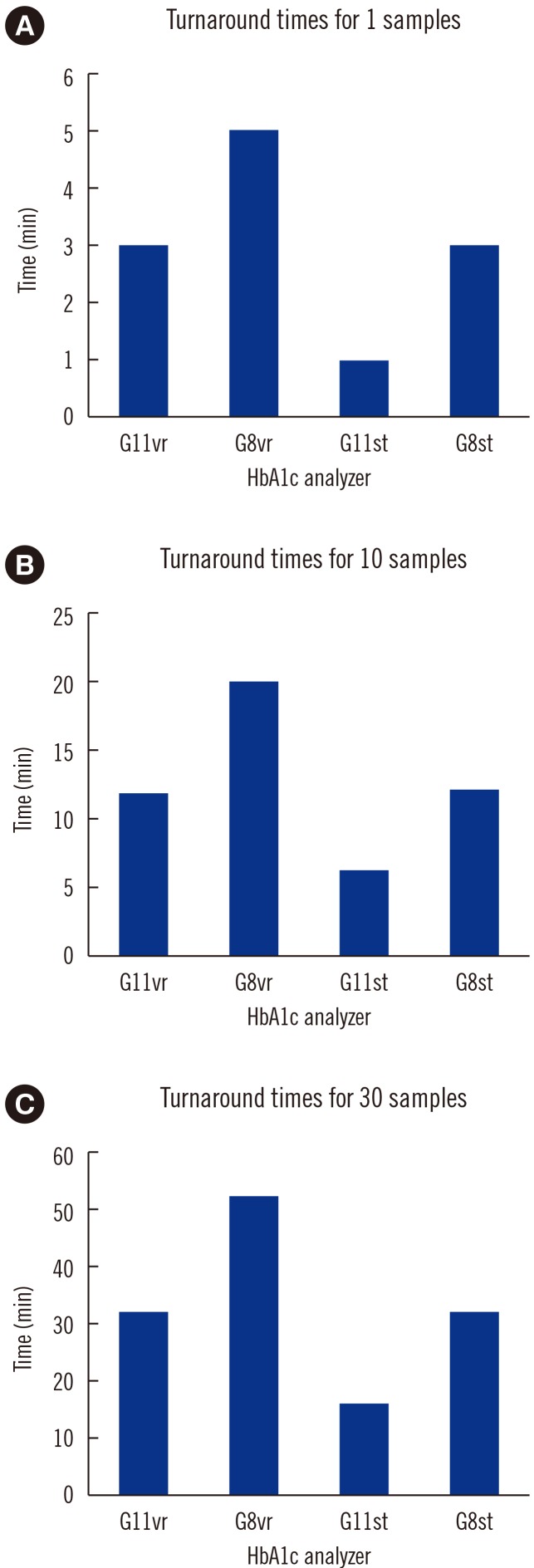
Repeatability (% CV) in measuring low- and high-concentration controls was 0.57% and 0.35%, respectively; within-laboratory precision was 0.86% and 0.69%, respectively. In a linearity test, the coefficient of determination was 0.9999 (measurement range: 3.64% to 18.59%) for HbA1c. The correlations between G11vr and other analyzers were weaker for flagged samples than for non-flagged samples. The carry-over effect was less than 0.4%. Turnaround time for a single sample was lower in G11vr (one minute) than in G8vr (1.6 minutes). For 15 samples with Hb variants, G11vr HbA1c results were more similar than those of other analyzers to HPLC-MS and CE results.
CONCLUSIONS
G11vr showed adequate performance and rapid turnaround time in measuring HbA1c.
Keyword
MeSH Terms
Figure
Reference
-
1. Weykamp C. HbA1c: a review of analytical and clinical aspects. Ann Lab Med. 2013; 33:393–400. PMID: 24205486.2. Korea national health and nutritional examination survey 2016. Updated on Jan 2018. https://knhanes.cdc.go.kr/knhanes/sub04/sub04_03.do?classType=7.3. Kwon SS, Kwon JY, Park YW, Kim YH, Lim JB. HbA1c for diagnosis and prognosis of gestational diabetes mellitus. Diabetes Res Clin Pract. 2015; 110:38–43. PMID: 26344325.4. Nishimura T, Meguro S, Sekioka R, Tanaka K, Saisho Y, Irie J, et al. A reduction of HbA1c after 3 months predicts 2-year responsiveness to sitagliptin treatment. Intern Med. 2015; 54:2981–2989. PMID: 26631880.5. Jeon Y, Han M, Lee K, Chang HE, Park KU, Song J. Performance evaluation of the CAPILLARYS 2 FLEX Piercing Analyzer for HbA1c determination. Lab Med Online. 2013; 3:221–226.6. Chung S, Jun SH, Song WH, Song J. Six years' experience of accuracy-based proficiency testing for HbA1c in Korea. J Lab Med Qual Assur. 2015; 37:92–100.7. Bouzid K, Ahmed HB, Kalai E, Blibeche S, Couque N, Khiari K, et al. Prevalence of hemoglobin variants in a diabetic population at high risk of hemoglobinopathies and optimization of HbA1c monitoring by incorporating HPLC in the laboratory workup. Libyan J Med. 2014; 9:25768.8. Koga M, Kurebayashi S, Murai J, Saito H, Miyazaki A. Degree of discrepancy between HbA1c and glycemia in variant hemoglobin is smaller when HbA1c is measured by new-type Arkray HPLC compared with old-type HPLC. Clin Biochem. 2014; 47:123–125. PMID: 24128409.9. CLSI. Evaluation of precision of quantitative measurement procedures; approved guideline. CLSI EP05-A3. 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute;2014.10. CLSI. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; approved guideline. CLSI EP06-A. Wayne, PA: Clinical and Laboratory Standards Institute;2003.11. CLSI. Measurement procedure comparison and bias estimation using patient samples; approved guideline. CLSI EP9-A3. 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute;2002.12. Wu X, Chao Y, Wan Z, Wang Y, Ma Y, Ke P, et al. A comparative evaluation of the analytical performances of Capillarys 2 Flex Piercing, Tosoh HLC-723 G8, Premier Hb9210, and Roche Cobas c501 Tina-quant Gen 2 analyzers for HbA1c determination. Biochem Med (Zagreb). 2016; 26:353–364. PMID: 27812304.13. Nasir NM, Thevarajah M, Yean CY. Hemoglobin variants detected by hemoglobin A1c (HbA1c) analysis and the effects on HbA1c measurements. Int J Diabetes Dev Ctries. 2010; 30:86–90. PMID: 20535312.14. World Health Organization. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation, 2011. WHO;Updated on Oct 2018. Available from: http://www.who.int/cardiovascular_diseases/report-hba1c_2011_edited.pdf.15. Yun YM, Ji M, Ko DH, Chun S, Kwon GC, Lee K, et al. Hb variants in Korea: effect on HbA1c using five routine methods. Clin Chem Lab Med. 2017; 55:1234–1242. PMID: 28107170.16. Xu A, Chen W, Xia Y, Zhou Y, Ji L. Effects of common hemoglobin variants on HbA1c measurements in China: results for alpha- and beta-globin variants measured by six methods. Clin Chem Lab Med. 2018; 56:1353–1361. PMID: 29626415.17. Jisuk Woo, Levinson JD. Diversity, dialogue, and deliberation: an empirical investigation of age, gender, and meaningful decision-making in Korean juries. Asian-Pacific L Policy J. 2017; 19:1:23–48.18. American Diabetes Association. (6) Glycemic targets. Diabetes Care. 2015; 38:S33–S40. PMID: 25537705.19. Bonadonna RC, Yale JF, Brulle-Wohlhueter C, Boëlle-Le Corfec E, Choudhary P, Bailey TS. Hypoglycaemia as a function of HbA1c in type 2 diabetes: Insulin glargine 300 U/mL in a patient-level pooled analysis of EDITION 1, 2 and 3. Diabetes Obes Metab. 2018.20. Doggui R, Abdelhafidh Sahli C, Aissa WL, Hammami M, Ben Sedrine M, Mahjoub R, et al. Capillarys 2 Flex Piercing: analytical performance assessment according to CLSI protocols for HbA1c quantification. Electrophoresis. 2017; 38:2210–2218. PMID: 28543614.21. Müller-Wieland D, Merkel M, Hamann A, Siegel E, Ottillinger B, Woker R, et al. Survey to estimate the prevalence of type 2 diabetes mellitus in hospital patients in Germany by systematic HbA1c measurement upon admission. Int J Clin Pract. 2018; 72:e13273. PMID: 30295392.22. Sánchez-González CM, Castillo-Mora A, Alvarado-Maldonado IN, Ortega-González C, Martínez-Cruz N, Arce-Sánchez L, et al. Reference intervals for hemoglobin A1c (HbA1c) in healthy Mexican pregnant women: a cross-sectional study. BMC Pregnancy Childbirth. 2018; 18:424. PMID: 30373541.23. Wang Y, Peng W, Tang J, Dong L, Gu C, Zhang X, et al. Verification of a novel point-of-care HbA1c device in real world clinical practice by comparison to three high performance liquid chromatography instruments. Biochem Med (Zagreb). 2018; 28:020705. PMID: 29666558.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Analytical Performance of an Automated Glycated Hemoglobin Analyzer, HLC-723 G11
- Evaluation of the Tosoh HLC-723 G7 Hemoglobin A1c Analyzer
- Evaluation of HLC-723 G7 Hemoglobin A1c Autoanalyzer
- Evaluation of the Tosoh HLC-723 G8 HbA1c Autoanalyzer
- Evaluation of Hemoglobin A1c on the Cobas Integra 800 Immunoassay and Tosoh HLC-723 G8 HPLC Analyzer