Lab Anim Res.
2018 Mar;34(1):1-10. 10.5625/lar.2018.34.1.1.
Establishment of a chronic obstructive pulmonary disease mouse model based on the elapsed time after LPS intranasal instillation
- Affiliations
-
- 1Department of Nursing, Dongshin University, Naju, Korea. dhj1221@hanmail.net
- 2Department of Pharmacy, College of Pharmacy, Mokpo National University, Muan, Korea.
- 3College of Veterinary Medicine, Chonnam National University, Gwangju, Korea.
- 4Department of Physical Therapy, Dongshin University, Naju, Korea. kykim@dsu.ac.kr
- KMID: 2430880
- DOI: http://doi.org/10.5625/lar.2018.34.1.1
Abstract
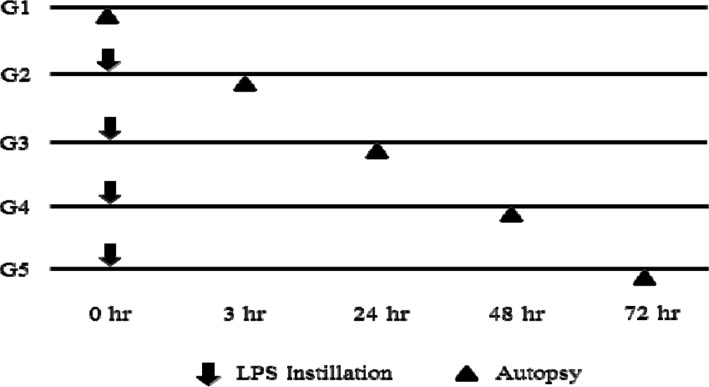
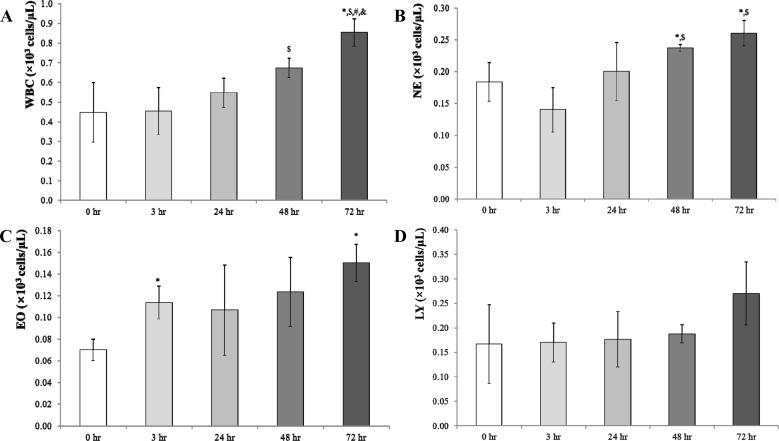
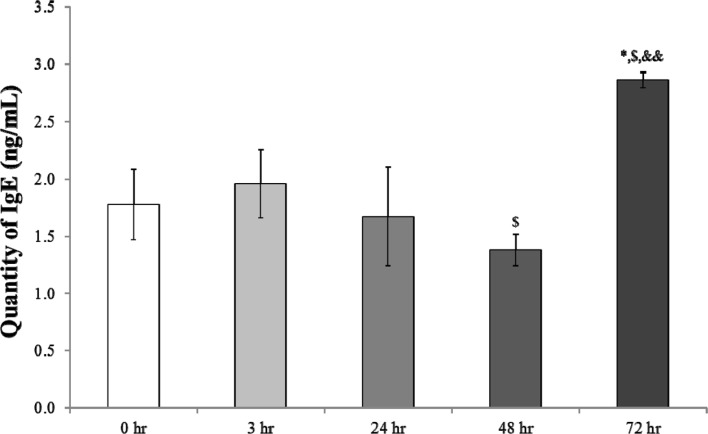
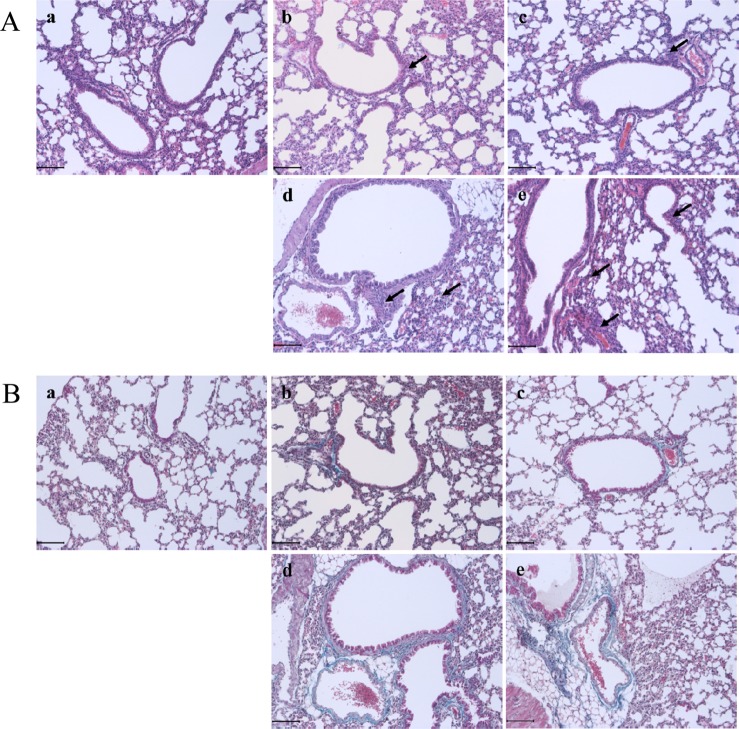
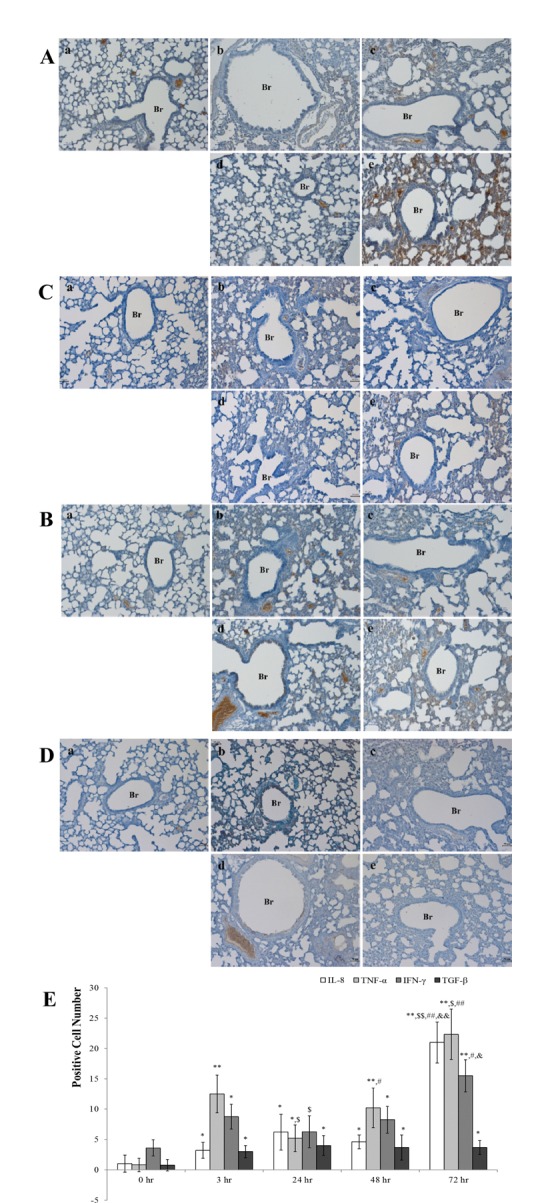
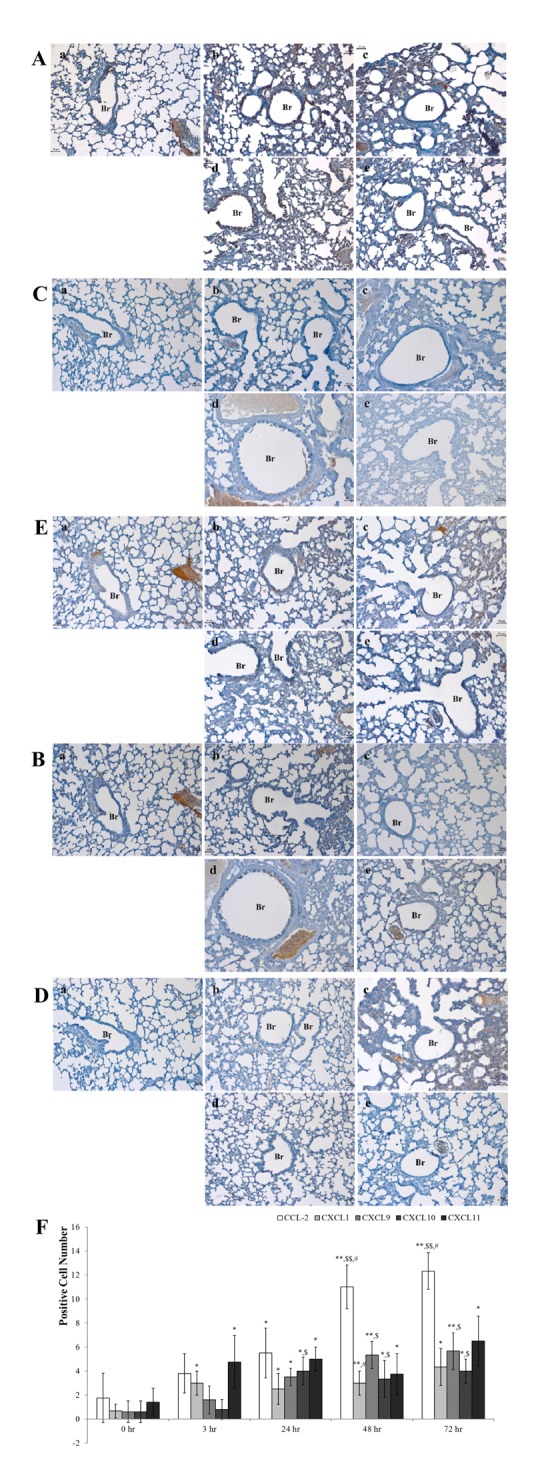
- Chronic obstructive pulmonary disease (COPD) was the 3rd leading cause of death in 2012 worldwide. It is particularly severe in the elderly, who are at risk of death by coughing, mucous hypersecretion, and finally breathlessness. Recently, anti-COPD drug development has increased, and many animal screening systems have been studied. Tobacco smoke animal models are the best known animal screening system, but have several preparation requirements, such as a tobacco smoke generator and a separate facility to prevent smoke release. Accordingly, we evaluated the properties of a lipopolysaccharide (LPS) murine model for COPD screening and the effect of the time elapsed from 0 to 72 hr after LPS intranasal instillation on various biomarkers of COPD severity, such as WBC and neutrophils in bronchoalveolar fluid (BALF), IgE in serum, histopathology in the lung, and cytokines (IL-8, TNF-α, IFN-γ, and TGF-β) and chemokines (CCL-2, CXCL1, CXCL9, CXCL10, and CXCL11) in the respiratory system. Although from 48 hr after LPS treatment several factors which could be evaluated as biomarkers for COPD establishment such as WBC and neutrophil in BALF, IgE in serum, cytokines (IL-8, TNF-α, and IFN-γ), and chemokines (CCL-2, CXCL1, CXCL9, CXCL10, and CXCL11) increased at 72 hr the increment of important factors for COPD establishment such as IgE, fibrosis in the lung, and cytokines (IL-8, TNF-α, and IFN-γ) was more clear. Based on our results, we concluded that the optimal time after LPS intranasal instillation is 72 hr.
Keyword
MeSH Terms
Figure
Reference
-
1. World Health Organization. November 2016 Chronic obstructive pulmonary disease (COPD). 2016. Fact sheet.2. World Health Organization. The top 10 causes of death. 2014. Fact sheet No310.3. Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Paré PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004; 350(26):2645–2653. PMID: 15215480.
Article4. Barnes PJ. The cytokine network in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2009; 41(6):631–638. PMID: 19717810.
Article5. Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009; 4:435–459. PMID: 18954287.
Article6. Churg A, Cosio M, Wright JL. Mechanisms of cigarette smoke-induced COPD: insights from animal models. Am J Physiol Lung Cell Mol Physiol. 2008; 294(4):L612–L631. PMID: 18223159.
Article7. Maes T, Provoost S, Lanckacker EA, Cataldo DD, Vanoirbeek JA, Nemery B, Tournoy KG, Joos GF. Mouse models to unravel the role of inhaled pollutants on allergic sensitization and airway inflammation. Respir Res. 2010; 11(1):7. PMID: 20092634.
Article8. Birrell MA, Wong S, Hele DJ, McCluskie K, Hardaker E, Belvisi MG. Steroid-resistant inflammation in a rat model of chronic obstructive pulmonary disease is associated with a lack of nuclear factor-kappaB pathway activation. Am J Respir Crit Care Med. 2005; 172(1):74–84. PMID: 15805185.9. Håkansson HF, Smailagic A, Brunmark C, Miller-Larsson A, Lal H. Altered lung function relates to inflammation in an acute LPS mouse model. Pulm Pharmacol Ther. 2012; 25(5):399–406. PMID: 22975080.
Article10. Bang MA, Seo JH, Seo JW, Jo GH, Jung SK, Yu R, Park DH, Park SJ. Bacillus subtilis KCTC 11782BP-produced alginate oligosaccharide effectively suppresses asthma via T-helper cell type 2-related cytokines. PLos One. 2015; 10(7):e0130510. PMID: 26132168.
Article11. Gompertz S, O'Brien C, Bayley DL, Hill SL, Stockley RA. Changes in bronchial inflammation during acute exacerbations of chronic bronchitis. Eur Respir J. 2001; 17(6):1112–1119. PMID: 11491152.
Article12. Samaha HMS, Elsaid AR, NasrEldin E. Total serum IgE level in COPD patients. Egypt J Chest Dis Tuberc. 2015; 64(3):573–577.
Article13. America's Biopharmaceutical Research Companies 2012. Medicines in development COPD;2012. Report.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Bone Marrow Mononuclear Cells on Lung Regeneration and Apoptosis in a Simple Model of Pulmonary Emphysema
- Cor Pulmonale with Particular Reference to Chronic Obstructive Pulmonary Disease and Pulmonary Tuberculosis
- Structural Equation Modeling on Successful Aging in Elders with Chronic Obstructive Pulmonary Disease Based on Selection-Optimization-Compensation Strategy
- Management of Chronic Obstructive Pulmonary Disease
- Pathophysiology of Chronic Obstructive Pulmonary Disease