Ann Dermatol.
2019 Feb;31(1):22-28. 10.5021/ad.2019.31.1.22.
Efficacy of Bacteriophages in Propionibacterium acnes-Induced Inflammation in Mice
- Affiliations
-
- 1Department of Dermatology, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu, Korea. weonju@knu.ac.kr
- 2Department of Microbiology, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu, Korea.
- KMID: 2430797
- DOI: http://doi.org/10.5021/ad.2019.31.1.22
Abstract
- BACKGROUND
Bacteriophages have been introduced as living drugs for infectious diseases; thus, they may provide an alternative to conventional acne therapeutics in patients with non-responsive acne.
OBJECTIVE
We investigated the effect of bacteriophages using an acne mouse model with Propionibacterium acnes-induced inflammatory nodules by clinical examination, pathology, and immunohistochemical analysis.
METHODS
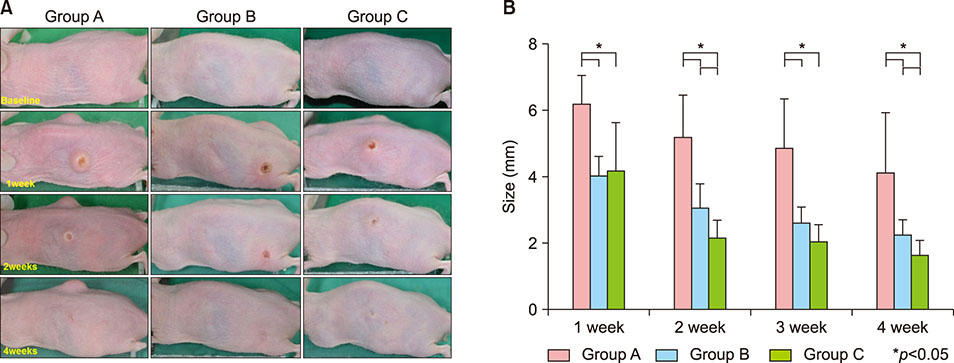
A human-isolated P. acnes suspension (10â¹ colony forming units/µl) was injected into the backs of HR-1 mice. Group A was used as a control, Group B was injected on the back with P. acnes 4 weeks following the initial P. acnes suspension injection, and group C was injected on the back with P. acnes and bacteriophages 4 weeks following the initial P. acnes suspension injection. Clinical and histopathological evaluations were performed.
RESULTS
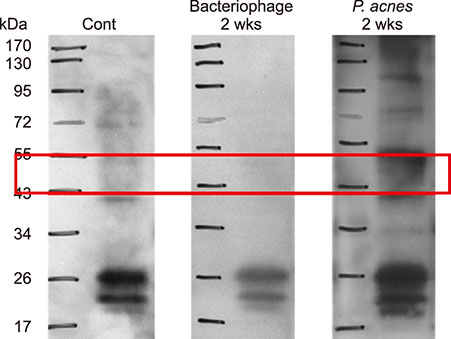
Inflammatory nodule size decreased with time in all groups. Group C showed the greatest decrease in size, followed by group B and group A. The histopathological findings showed a decrease in epidermal thickness and the number and size of microcomedone-like cysts in groups B and C compared to group A. Immunohistochemistry revealed similar expression of integrin α6, the epidermal proliferation marker, infiltration of CD4/CD8 T cells and neutrophils, and expression of myeloperoxidase, interleukin-1β, toll-like receptor-2, LL-37, and matrix metalloproteinase-2/3/9 in all three groups.
CONCLUSION
Using an acne mouse model with P. acnes-induced inflammatory nodules, we demonstrate that bacteriophages may constitute an alternative to conventional acne therapies. However, additional studies are needed for human applications.
Keyword
MeSH Terms
Figure
Reference
-
1. Shaheen B, Gonzalez M. Acne sans P. acnes. J Eur Acad Dermatol Venereol. 2013; 27:1–10.2. Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J invest dermatol. 2003; 121:20–27.
Article3. Mirshahpanah P, Maibach HI. Models in acnegenesis. Cutan Ocul Toxicol. 2007; 26:195–202.
Article4. Jang YH, Lee KC, Lee SJ, Kim DW, Lee WJ. HR-1 mice: a new inflammatory acne mouse model. Ann Dermatol. 2015; 27:257–264.
Article5. Jończyk-Matysiak E, Weber-Dąbrowska B, Żaczek M, Międzybrodzki R, Letkiewicz S, Łusiak-Szelchowska M, et al. Prospects of phage application in the treatment of acne caused by Propionibacterium acnes. Front Microbiol. 2017; 8:164.
Article6. Jassim SA, Limoges RG. Natural solution to antibiotic resistance: bacteriophages ‘The Living Drugs’. World J Microbiol Biotechnol. 2014; 30:2153–2170.
Article7. Marples RR. The microflora of the face and acne lesions. J Invest Dermatol. 1974; 62:326–331.
Article8. Sandoval LF, Hartel JK, Feldman SR. Current and future evidence-based acne treatment: a review. Expert Opin Pharmacother. 2014; 15:173–192.
Article9. Bienenfeld A, Nagler AR, Orlow SJ. Oral antibacterial therapy for acne vulgaris: an evidence-based review. Am J Clin Dermatol. 2017; 18:469–490.
Article10. Vallerand IA, Lewinson RT, Farris MS, Sibley CD, Ramien ML, Bulloch AGM, et al. Efficacy and adverse events of oral isotretinoin for acne: a systematic review. Br J Dermatol. 2018; 178:76–85.
Article11. Nakatsuji T, Rasochova L, Huang CM. Vaccine therapy for P. acnes-associated diseases. Infect Disord Drug Targets. 2008; 8:160–165.
Article12. Nakatsuji T, Liu YT, Huang CP, Zoubouis CC, Gallo RL, Huang CM. Antibodies elicited by inactivated Propionibacterium acnes-based vaccines exert protective immunity and attenuate the IL-8 production in human sebocytes: relevance to therapy for acne vulgaris. J Invest Dermatol. 2008; 128:2451–2457.
Article13. Mussalem JS, Vasconcelos JR, Squaiella CC, Ananias RZ, Braga EG, Rodrigues MM, et al. Adjuvant effect of the Propionibacterium acnes and its purified soluble polysaccharide on the immunization with plasmidial DNA containing a Trypanosoma cruzi gene. Microbiol Immunol. 2006; 50:253–263.
Article14. Lodes MJ, Secrist H, Benson DR, Jen S, Shanebeck KD, Guderian J, et al. Variable expression of immunoreactive surface proteins of Propionibacterium acnes. Microbiology. 2006; 152:3667–3681.
Article15. Nakatsuji T, Liu YT, Huang CP, Zouboulis CC, Gallo RL, Huang CM. Vaccination targeting a surface sialidase of P. acnes: implication for new treatment of acne vulgaris. PLoS One. 2008; 3:e1551.
Article16. Brüggemann H. Insights in the pathogenic potential of Propionibacterium acnes from its complete genome. Semin Cutan Med Surg. 2005; 24:67–72.
Article17. Nakatsuji T, Tang DC, Zhang L, Gallo RL, Huang CM. Propionibacterium acnes CAMP factor and host acid sphingomyelinase contribute to bacterial virulence: potential targets for inflammatory acne treatment. PLoS One. 2011; 6:e14797.
Article18. Lo CW, Lai YK, Liu YT, Gallo RL, Huang CM. Staphylococcus aureus hijacks a skin commensal to intensify its virulence: immunization targeting beta-hemolysin and CAMP factor. J Invest Dermatol. 2011; 131:401–409.
Article19. Ozlu E, Karadag AS, Ozkanli S, Oguztuzun S, Kilic M, Zemheri E, et al. Comparison of TLR-2, TLR-4, and antimicrobial peptide levels in different lesions of acne vulgaris. Cutan Ocul Toxicol. 2016; 35:300–309.
Article20. Górski A, Dąbrowska K, Hodyra-Stefaniak K, Borysowski J, Międzybrodzki R, Weber-Dąbrowska B. Phages targeting infected tissues: novel approach to phage therapy. Future Microbiol. 2015; 10:199–204.
Article21. Katsuta Y, Iida T, Inomata S, Denda M. Unsaturated fatty acids induce calcium influx into keratinocytes and cause abnormal differentiation of epidermis. J Invest Dermatol. 2005; 124:1008–1013.
Article22. Borovaya A, Dombrowski Y, Zwicker S, Olisova O, Ruzicka T, Wolf R, et al. Isotretinoin therapy changes the expression of antimicrobial peptides in acne vulgaris. Arch Dermatol Res. 2014; 306:689–700.
Article23. Brüggemann H, Lood R. Bacteriophages infecting propionibacterium acnes. Biomed Res Int. 2013; 2013:705741.24. Brown TL, Petrovski S, Dyson ZA, Seviour R, Tucci J. The formulation of bacteriophage in a semi solid preparation for control of Propionibacterium acnes growth. PLoS One. 2016; 10:e0151184.
Article25. Esteban PP, Alves DR, Enright MC, Bean JE, Gaudion A, Jenkins AT, et al. Enhancement of the antimicrobial properties of bacteriophage-K via stabilization using oil-in-water nano-emulsions. Biotechnol Prog. 2014; 30:932–944.
Article26. Chen LK, Liu LY, Hu A, Chang KC, Lin NT, Lai MJ, et al. Potential of bacteriophage ΦAB2 as an environmental biocontrol agent for the control of multidrug-resistant Acinetobacter baumannii. BMC Microbiol. 2013; 13:154.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Red or Infrared Light-Emitting Diodes in a Mouse Model of Propionibacterium acnes-Induced Inflammation
- A study on the chemotactic activity of the peripheral blood neutrop- hils in acne patients to the cytosol antigen of propionibacterium acnes
- A Case of Propionibacterium acnes Endophthalmitis after Extracapsular Cataract Extraction and Posterior Chamber Lens Implantation
- Study of the Photoinactivation Effect on Propionibacterium acnes after Light Irradiation with Variable Wavelengths
- Effects of Magnesium Ascorbyl Phosphate on the Expression of Inflammatory Biomarkers after Treatment of Cultured Sebocytes with Propionibacterium acnes or Ultraviolet B Radiation