Linked Color Imaging and Blue Laser Imaging for Upper Gastrointestinal Screening
- Affiliations
-
- 1Division of Gastroenterology, Department of Medicine, Jichi Medical University, Shimotsuke, Japan. osawa@jichi.ac.jp
- 2Department of Medicine, Department of Surgery, Jichi Medical University, Shimotsuke, Japan.
- KMID: 2430392
- DOI: http://doi.org/10.5946/ce.2018.132
Abstract
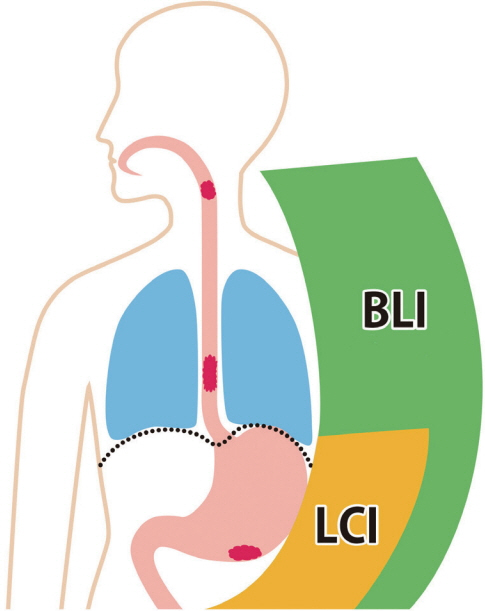
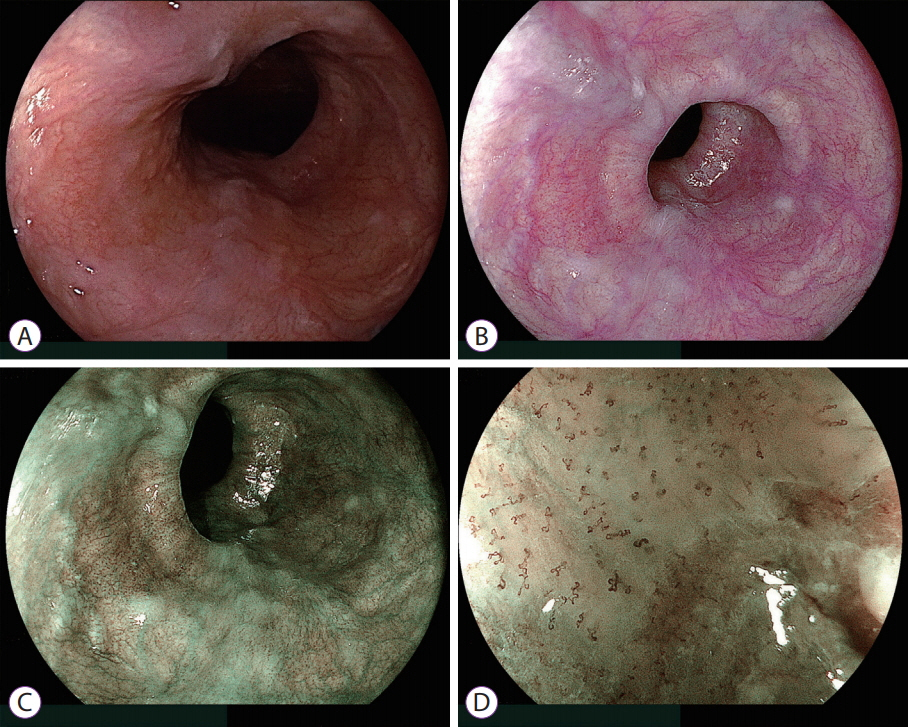
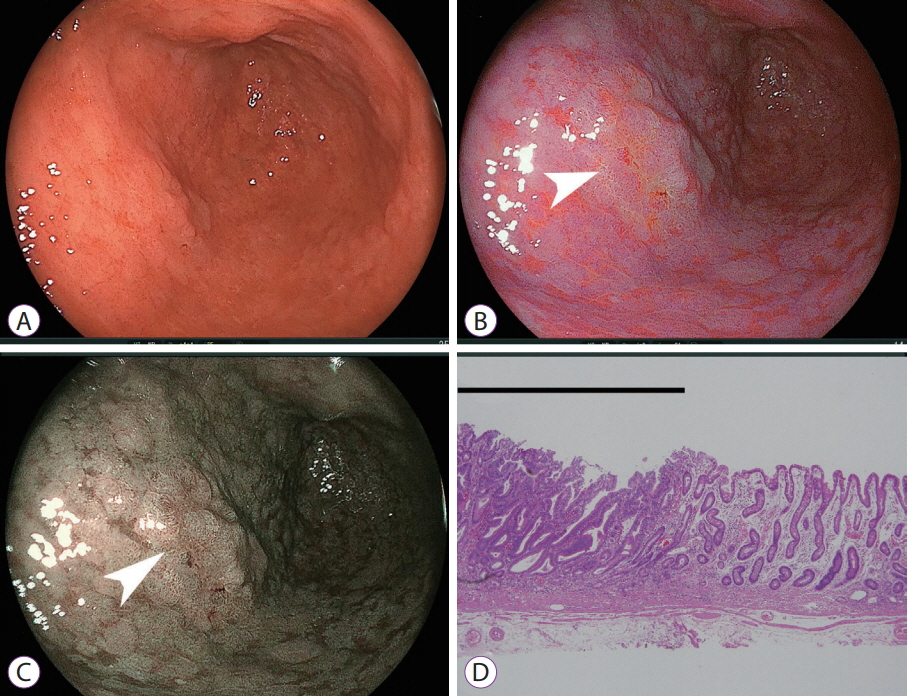
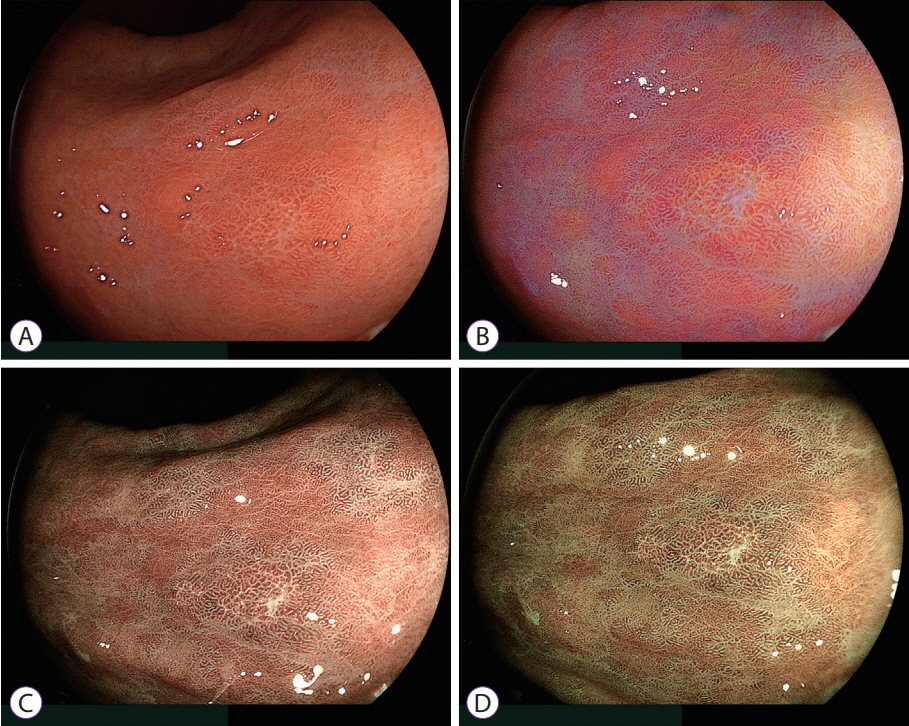
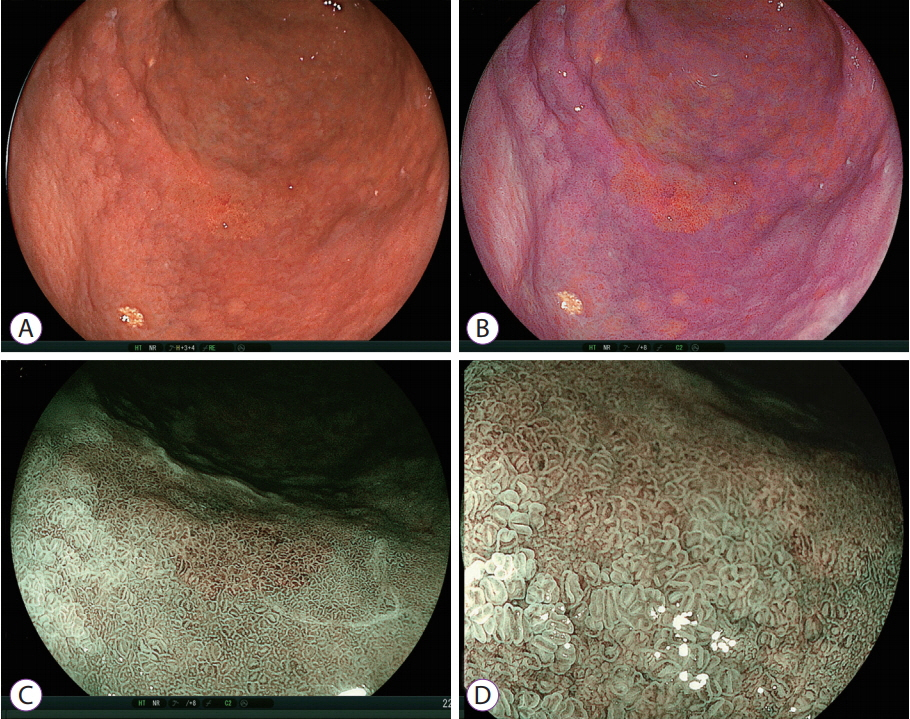
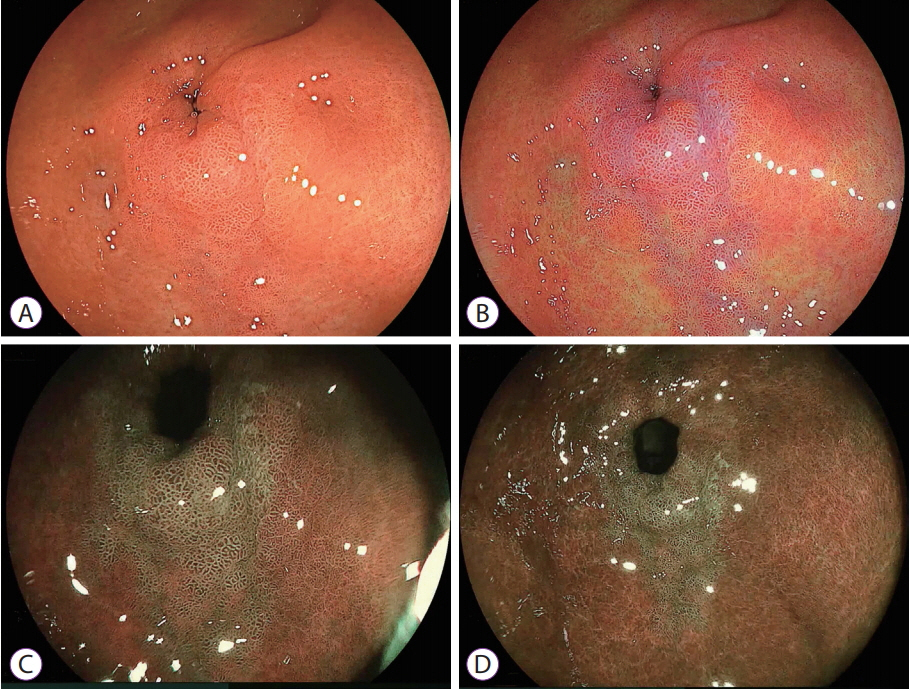
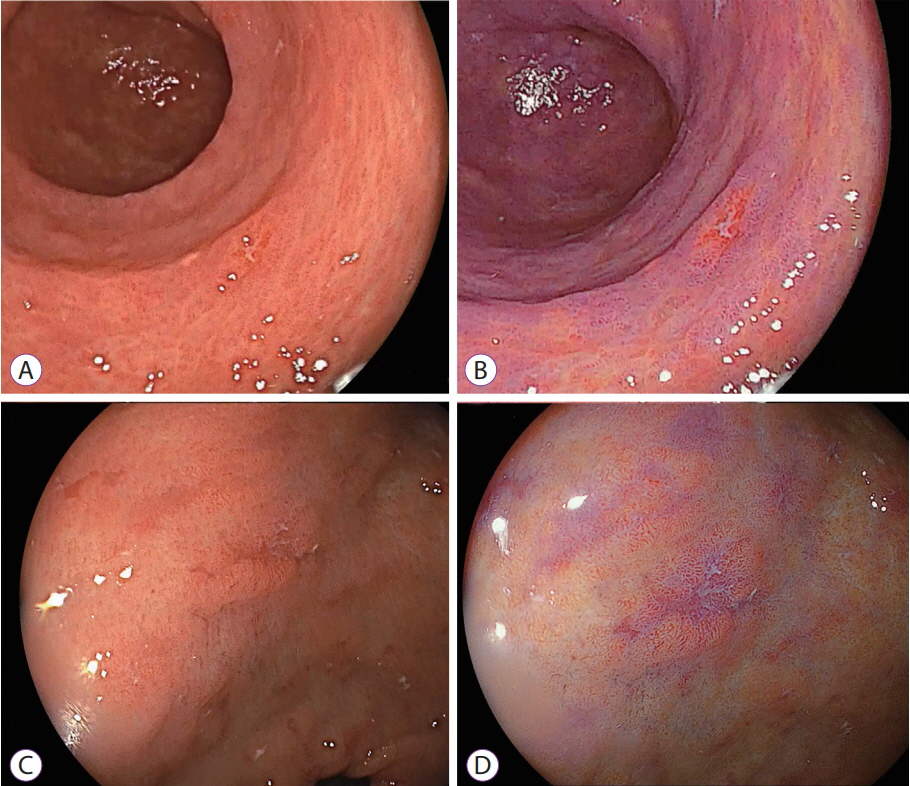
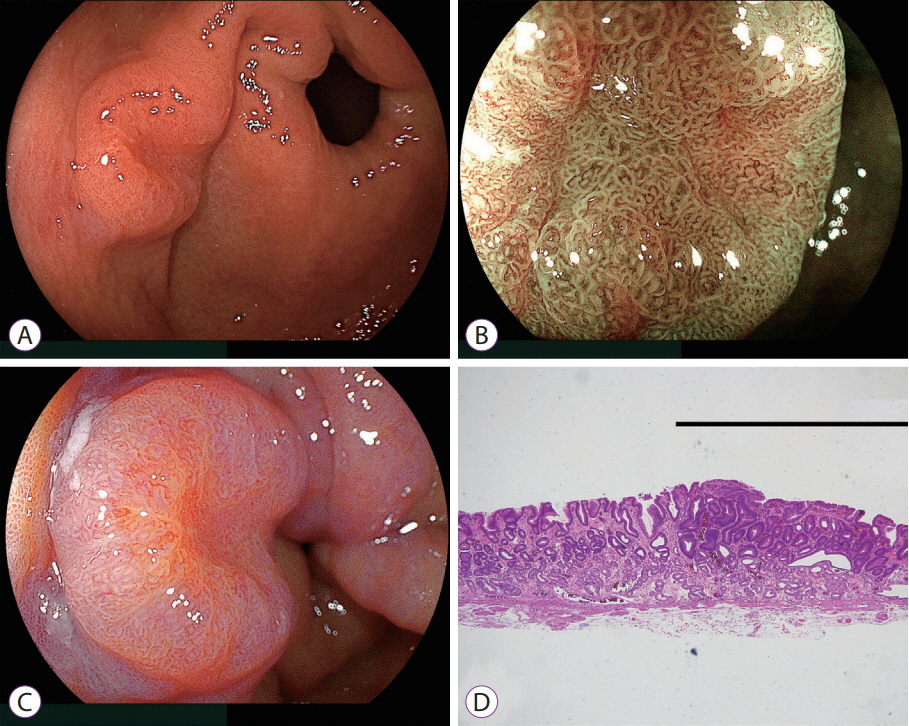
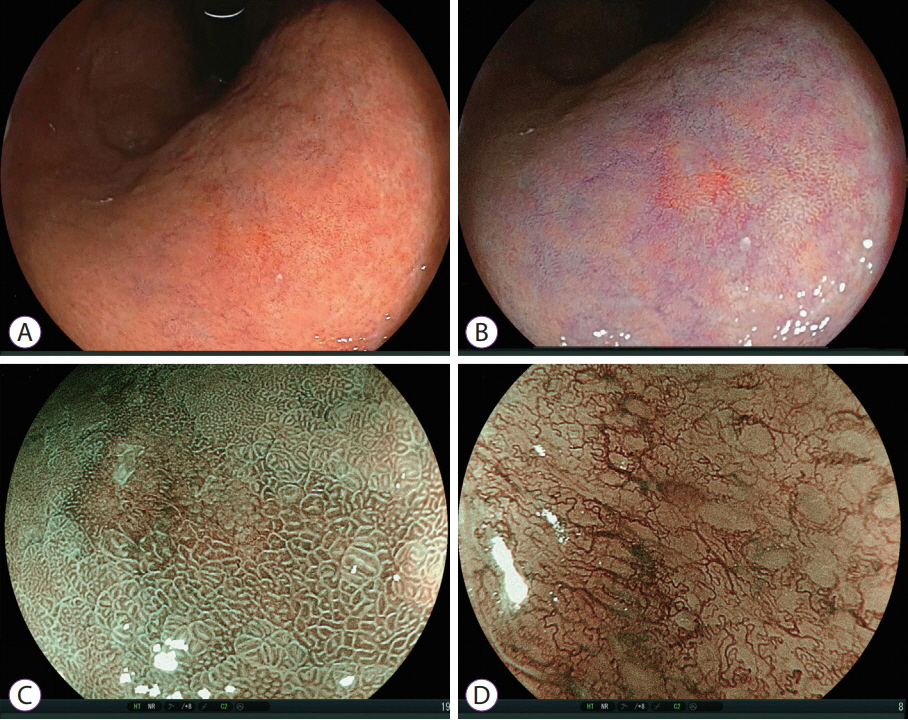
- White light imaging (WLI) may not reveal early upper gastrointestinal cancers. Linked color imaging (LCI) produces bright images in the distant view and is performed for the same screening indications as WLI. LCI and blue laser imaging (BLI) provide excellent visibility of gastric cancers in high color contrast with respect to the surrounding tissue. The characteristic purple and green color of metaplasias on LCI and BLI, respectively, serve to increase the contrast while visualizing gastric cancers regardless of a history of Helicobacter pylori eradication. LCI facilitates color-based recognition of early gastric cancers of all morphological types, including flat lesions or those in an H. pylori-negative normal background mucosa as well as the diagnosis of inflamed mucosae including erosions. LCI reveals changes in mucosal color before the appearance of morphological changes in various gastric lesions. BLI is superior to LCI in the detection of early esophageal cancers and abnormal findings of microstructure and microvasculature in close-up views of upper gastrointestinal cancers. Excellent images can also be obtained with transnasal endoscopy. Using a combination of these modalities allows one to obtain images useful for establishing a diagnosis. It is important to observe esophageal cancers (brown) using BLI and gastric cancers (orange) surrounded by intestinal metaplasia (purple) and duodenal cancers (orange) by LCI.
MeSH Terms
Figure
Cited by 5 articles
-
Linked Color Imaging Demonstrates Characteristic Findings in Semi-Pedunculated Gastric Adenocarcinoma in
Helicobacter pylori -Negative Normal Mucosa
Yuji Hiraoka, Yoshimasa Miura, Hiroyuki Osawa, Mio Sakaguchi, Masato Tsunoda, Alan Kawarai Lefor, Hironori Yamamoto
Clin Endosc. 2021;54(1):136-138. doi: 10.5946/ce.2020.059.Clinical Applications of Linked Color Imaging and Blue Laser/Light Imaging in the Screening, Diagnosis, and Treatment of Superficial Colorectal Tumors
Taku Sakamoto, Hourin Cho, Yutaka Saito
Clin Endosc. 2021;54(4):488-493. doi: 10.5946/ce.2021.157.Application of Current Image-Enhanced Endoscopy in Gastric Diseases
Wansik Lee
Clin Endosc. 2021;54(4):477-487. doi: 10.5946/ce.2021.160.New Diagnostic Approach for Esophageal Squamous Cell Neoplasms Using Linked Color Imaging and Blue Laser Imaging Combined with Iodine Staining
Masato Tsunoda, Yoshimasa Miura, Hiroyuki Osawa, Tsevelnorov Khurelbaatar, Mio Sakaguchi, Hisashi Fukuda, Alan Kawarai Lefor, Hironori Yamamoto
Clin Endosc. 2019;52(5):497-501. doi: 10.5946/ce.2018.195.Current Endoscopy Training in Korea and Future Aspects
Young-Eun Joo
Korean J Gastroenterol. 2022;80(5):207-210. doi: 10.4166/kjg.2022.122.
Reference
-
1. Osawa H, Yoshizawa M, Yamamoto H, et al. Optimal band imaging system can facilitate detection of changes in depressed-type early gastric cancer. Gastrointest Endosc. 2008; 67:226–234.
Article2. Yoshizawa M, Osawa H, Yamamoto H, et al. Diagnosis of elevated-type early gastric cancers by the optimal band imaging system. Gastrointest Endosc. 2009; 69:19–28.
Article3. Yao K, Oishi T, Matsui T, Yao T, Iwashita A. Novel magnified endoscopic findings of microvascular architecture in intramucosal gastric cancer. Gastrointest Endosc. 2002; 56:279–284.
Article4. Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004; 36:1080–1084.
Article5. Kaise M, Kato M, Urashima M, et al. Magnifying endoscopy combined with narrow-band imaging for differential diagnosis of superficial depressed gastric lesions. Endoscopy. 2009; 41:310–315.
Article6. Kaneko K, Oono Y, Yano T, et al. Effect of novel bright image enhanced endoscopy using blue laser imaging (BLI). Endosc Int Open. 2014; 2:E212–E219.
Article7. Osawa H, Yamamoto H. Present and future status of flexible spectral imaging color enhancement and blue laser imaging technology. Dig Endosc. 2014; 26 Suppl 1:105–115.
Article8. Osawa H, Yamamoto H, Miura Y, et al. Blue laser imaging provides excellent endoscopic images of upper gastrointestinal lesions. Video Journal and Encyclopedia of GI Endoscopy. 2014; 1:607–610.
Article9. Mouri R, Yoshida S, Tanaka S, Oka S, Yoshihara M, Chayama K. Evaluation and validation of computed virtual chromoendoscopy in early gastric cancer. Gastrointest Endosc. 2009; 69:1052–1058.
Article10. Ono H. Early gastric cancer: diagnosis, pathology, treatment techniques and treatment outcomes. Eur J Gastroenterol Hepatol. 2006; 18:863–866.
Article11. Muto M, Minashi K, Yano T, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010; 28:1566–1572.
Article12. Ono S, Abiko S, Kato M. Linked color imaging enhances gastric cancer in gastric intestinal metaplasia. Dig Endosc. 2017; 29:230–231.
Article13. Fukuda H, Miura Y, Hayashi Y, et al. Linked color imaging technology facilitates early detection of flat gastric cancers. Clin J Gastroenterol. 2015; 8:385–389.
Article14. Kanzaki H, Takenaka R, Kawahara Y, et al. Linked color imaging (LCI), a novel image-enhanced endoscopy technology, emphasizes the color of early gastric cancer. Endosc Int Open. 2017; 5:E1005–E1013.
Article15. Diao W, Huang X, Shen L, Zeng Z. Diagnostic ability of blue laser imaging combined with magnifying endoscopy for early esophageal cancer. Dig Liver Dis. 2018; 50:1035–1040.
Article16. Iwashita C, Miura Y, Osawa H, et al. Laser imaging facilitates early detection of synchronous adenocarcinomas in patients with Barrett’s esophagus. Clin Endosc. 2017; 50:81–86.
Article17. Tomie A, Dohi O, Yagi N, et al. Blue laser imaging-bright improves endoscopic recognition of superficial esophageal squamous cell carcinoma. Gastroenterol Res Pract. 2016; 2016:6140854.
Article18. Osawa H, Yamamoto H, Yamada N, et al. Diagnosis of endoscopic Barrett’s esophagus by transnasal flexible spectral imaging color enhancement. J Gastroenterol. 2009; 44:1125–1132.
Article19. Takeda T, Nagahara A, Ishizuka K, et al. Improved visibility of Barrett’s esophagus with linked color imaging: inter- and intra-rater reliability and quantitative analysis. Digestion. 2018; 97:183–194.
Article20. Raftopoulos SC, Segarajasingam DS, Burke V, Ee HC, Yusoff IF. A cohort study of missed and new cancers after esophagogastroduodenoscopy. Am J Gastroenterol. 2010; 105:1292–1297.
Article21. Abe S, Oda I, Minagawa T, et al. Metachronous gastric cancer following curative endoscopic resection of early gastric cancer. Clin Endosc. 2018; 51:253–259.
Article22. Kaminishi M, Yamaguchi H, Nomura S, et al. Endoscopic classification of chronic gastritis based on a pilot study by the research society for gastritis. Dig Endosc. 2002; 14:138–151.
Article23. Eda A, Osawa H, Yanaka I, et al. Expression of homeobox gene CDX2 precedes that of CDX1 during the progression of intestinal metaplasia. J Gastroenterol. 2002; 37:94–100.
Article24. Osawa H, Inoue F, Yoshida Y. Inverse relation of serum Helicobacter pylori antibody titres and extent of intestinal metaplasia. J Clin Pathol. 1996; 49:112–115.
Article25. Chen H, Liu Y, Lu Y, et al. Ability of blue laser imaging with magnifying endoscopy for the diagnosis of gastric intestinal metaplasia. Lasers Med Sci. 2018; 33:1757–1762.
Article26. Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gan. 1968; 59:251–258.27. Matsukura N, Suzuki K, Kawachi T, et al. Distribution of marker enzymes and mucin in intestinal metaplasia in human stomach and relation to complete and incomplete types of intestinal metaplasia to minute gastric carcinomas. J Natl Cancer Inst. 1980; 65:231–240.28. Kohli Y, Pfeiffer CJ, Kutty KP, Barrowman JA, Heughan C, Kepkay DL. Endoscopic diagnosis of intestinal metaplasia in Canada and Japan. J Clin Gastroenterol. 1981; 3 Suppl 1:29–33.
Article29. Yoshifuku Y, Sanomura Y, Oka S, et al. Evaluation of the visibility of early gastric cancer using linked color imaging and blue laser imaging. BMC Gastroenterol. 2017; 17:150.
Article30. Osawa H, Nakazato M, Date Y, et al. Impaired production of gastric ghrelin in chronic gastritis associated with Helicobacter pylori. J Clin Endocrinol Metab. 2005; 90:10–16.31. Osawa H, Kita H, Ohnishi H, et al. Helicobacter pylori eradication induces marked increase in H+/K+-adenosine triphosphatase expression without altering parietal cell number in human gastric mucosa. Gut. 2006; 55:152–157.
Article32. Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008; 372:392–397.
Article33. Kamada T, Hata J, Sugiu K, et al. Clinical features of gastric cancer discovered after successful eradication of Helicobacter pylori: results from a 9-year prospective follow-up study in Japan. Aliment Pharmacol Ther. 2005; 21:1121–1126.
Article34. Kobayashi M, Hashimoto S, Nishikura K, et al. Magnifying narrow-band imaging of surface maturation in early differentiated-type gastric cancers after Helicobacter pylori eradication. J Gastroenterol. 2013; 48:1332–1342.
Article35. Ito M, Tanaka S, Takata S, et al. Morphological changes in human gastric tumours after eradication therapy of Helicobacter pylori in a shortterm follow-up. Aliment Pharmacol Ther. 2005; 21:559–566.
Article36. Saka A, Yagi K, Nimura S. Endoscopic and histological features of gastric cancers after successful Helicobacter pylori eradication therapy. Gastric Cancer. 2016; 19:524–530.
Article37. Shi J, Jin N, Li Y, Wei S, Xu L. Clinical study of autofluorescence imaging combined with narrow band imaging in diagnosing early gastric cancer and precancerous lesions. J BUON. 2015; 20:1215–1222.38. Toma S, Osawa H, Yoshizawa M, et al. Diagnosis of small flat early gastric cancer by flexible spectral imaging color enhancement. Clin J Gastroenterol. 2010; 3:88–91.
Article39. Takahashi H, Miura Y, Osawa H, et al. Blue laser imaging with a small-caliber endoscope facilitates detection of early gastric cancer. Clin Endosc. 2018; Aug. 14. [Epub]. https://doi.org/10.5946/ce.2018.100.
Article40. Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012; 366:687–696.
Article41. Kaminski MF, Wieszczy P, Rupinski M, et al. Increased rate of adenoma detection associates with reduced risk of colorectal cancer and death. Gastroenterology. 2017; 153:98–105.
Article42. Johnson JC, DiSario JA, Grady WM. Surveillance and treatment of periampullary and duodenal adenomas in familial adenomatous polyposis. Curr Treat Options Gastroenterol. 2004; 7:79–89.
Article43. Honda T, Yamamoto H, Osawa H, et al. Endoscopic submucosal dissection for superficial duodenal neoplasms. Dig Endosc. 2009; 21:270–274.
Article44. Miura Y, Shinozaki S, Hayashi Y, Sakamoto H, Lefor AK, Yamamoto H. Duodenal endoscopic submucosal dissection is feasible using the pocket-creation method. Endoscopy. 2017; 49:8–14.
Article45. Binmoeller KF, Shah JN, Bhat YM, Kane SD. “Underwater” EMR of sporadic laterally spreading nonampullary duodenal adenomas (with video). Gastrointest Endosc. 2013; 78:496–502.
Article46. Hamada K, Takeuchi Y, Ishikawa H, et al. Safety of cold snare polypectomy for duodenal adenomas in familial adenomatous polyposis: a prospective exploratory study. Endoscopy. 2018; 50:511–517.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of linked color imaging for upper gastrointestinal disease: present and future
- Detection of Gastrointestinal Cancer using Linked Color Imaging and Blue Light Imaging
- Clinical Applications of Linked Color Imaging and Blue Laser/Light Imaging in the Screening, Diagnosis, and Treatment of Superficial Colorectal Tumors
- Blue Laser Imaging, Blue Light Imaging, and Linked Color Imaging for the Detection and Characterization of Colorectal Tumors
- Laser Imaging Facilitates Early Detection of Synchronous Adenocarcinomas in Patients with Barrett's Esophagus