Anat Cell Biol.
2018 Dec;51(4):292-298. 10.5115/acb.2018.51.4.292.
Amelioration of experimental autoimmune encephalomyelitis by Ishige okamurae
- Affiliations
-
- 1Department of Veterinary Anatomy, Veterinary Medical Research Institute, College of Veterinary Medicine, Jeju National University, Jeju, Korea. shint@jejunu.ac.kr
- 2Department of Veterinary Histology, Veterinary Medical Research Institute, College of Veterinary Medicine, Jeju National University, Jeju, Korea.
- KMID: 2430197
- DOI: http://doi.org/10.5115/acb.2018.51.4.292
Abstract
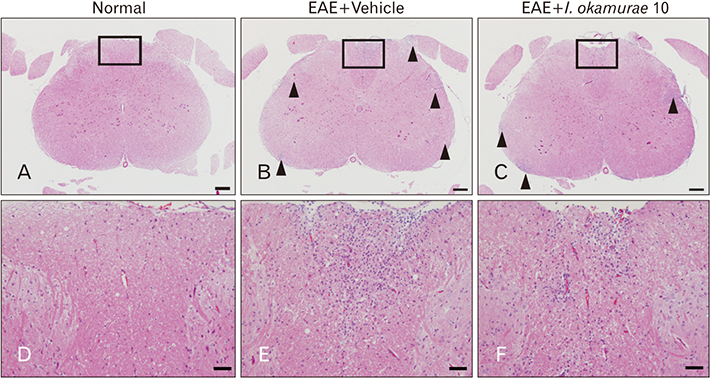
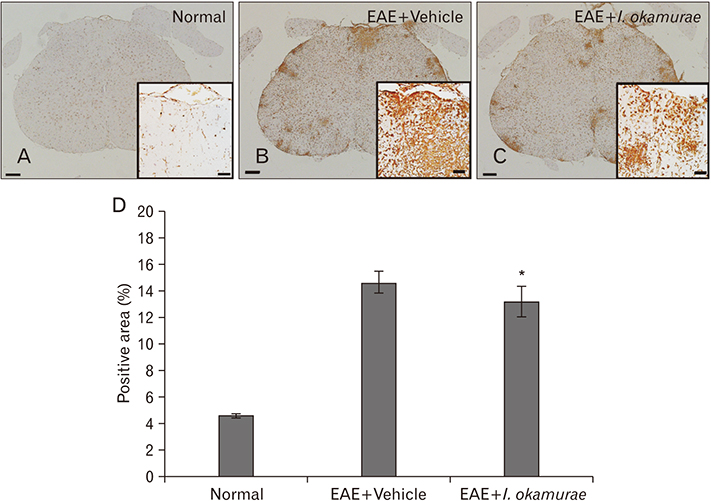
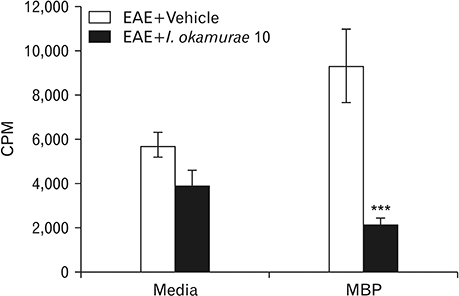
- Experimental autoimmune encephalomyelitis (EAE) is a T-cell-mediated autoimmune central nervous system disease characterized by inflammation with oxidative stress. The aim of this study was to evaluate an anti-inflammatory effect of Ishige okamurae on EAE-induced paralysis in rats. An ethanolic extract of I. okamurae significantly delayed the first onset and reduced the duration and severity of hind-limb paralysis. The neuropathological and immunohistochemical findings in the spinal cord were in agreement with these clinical results. T-cell proliferation assay revealed that the ethyl-acetate fraction of I. okamurae suppressed the proliferation of myelin basic protein reactive T cells from EAE affected rats. Flow cytometric analysis showed TCRαβ+ T cells was significantly reduced in the spleen of EAE rats with I. okamurae treatment with concurrent decrease of inflammatory mediators including tumor necrosis factor-α and cyclooxygenase-2. Collectively, it is postulated that I. okamurae ameliorates EAE paralysis with suppression of T-cell proliferation as well as decrease of pro-inflammatory mediators as far as rat EAE is concerned.
MeSH Terms
Figure
Reference
-
1. Shin T, Ahn M, Matsumoto Y. Mechanism of experimental autoimmune encephalomyelitis in Lewis rats: recent insights from macrophages. Anat Cell Biol. 2012; 45:141–148.
Article2. Tanuma N, Shin T, Kogure K, Matsumoto Y. Differential role of TNF-alpha and IFN-gamma in the brain of rats with chronic relapsing autoimmune encephalomyelitis. J Neuroimmunol. 1999; 96:73–79.3. Matsumoto Y, Kohyama K, Aikawa Y, Shin T, Kawazoe Y, Suzuki Y, Tanuma N. Role of natural killer cells and TCR gamma delta T cells in acute autoimmune encephalomyelitis. Eur J Immunol. 1998; 28:1681–1688.4. Wang P, Xie K, Wang C, Bi J. Oxidative stress induced by lipid peroxidation is related with inflammation of demyelination and neurodegeneration in multiple sclerosis. Eur Neurol. 2014; 72:249–254.
Article5. van Horssen J, Witte ME, Schreibelt G, de Vries HE. Radical changes in multiple sclerosis pathogenesis. Biochim Biophys Acta. 2011; 1812:141–150.
Article6. Ljubisavljevic S, Stojanovic I, Pavlovic D, Sokolovic D, Stevanovic I. Aminoguanidine and N-acetyl-cysteine supress oxidative and nitrosative stress in EAE rat brains. Redox Rep. 2011; 16:166–172.7. Lee IK, Kang JW. A check list of marine algae in Korea. Korean J Phycol. 1986; 1:311–325.8. Kim MM, Rajapakse N, Kim SK. Anti-inflammatory effect of Ishige okamurae ethanolic extract via inhibition of NF-kappaB transcription factor in RAW 264.7 cells. Phytother Res. 2009; 23:628–634.9. Heo SJ, Kim JP, Jung WK, Lee NH, Kang HS, Jun EM, Park SH, Kang SM, Lee YJ, Park PJ, Jeon YJ. Identification of chemical structure and free radical scavenging activity of diphlorethohydroxycarmalol isolated from a brown alga, Ishige okamurae. J Microbiol Biotechnol. 2008; 18:676–681.10. Zou Y, Qian ZJ, Li Y, Kim MM, Lee SH, Kim SK. Antioxidant effects of phlorotannins isolated from Ishige okamurae in free radical mediated oxidative systems. J Agric Food Chem. 2008; 56:7001–7009.11. Heo SJ, Cha SH, Kim KN, Lee SH, Ahn G, Kang DH, Oh C, Choi YU, Affan A, Kim D, Jeon YJ. Neuroprotective effect of phlorotannin isolated from Ishige okamurae against H2O2-induced oxidative stress in murine hippocampal neuronal cells, HT22. Appl Biochem Biotechnol. 2012; 166:1520–1532.12. Ahn M, Moon C, Yang W, Ko EJ, Hyun JW, Joo HG, Jee Y, Lee NH, Park JW, Ko RK, Kim GO, Shin T. Diphlorethohydroxycarmalol, isolated from the brown algae Ishige okamurae, protects against radiation-induced cell damage in mice. Food Chem Toxicol. 2011; 49:864–870.13. Shin T, Ahn M, Hyun JW, Kim SH, Moon C. Antioxidant marine algae phlorotannins and radioprotection: a review of experimental evidence. Acta Histochem. 2014; 116:669–674.
Article14. Kim H, Moon C, Park EJ, Jee Y, Ahn M, Wie MB, Shin T. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats treated with fucoidan. Phytother Res. 2010; 24:399–403.
Article15. Ahn M, Yang W, Kim H, Jin JK, Moon C, Shin T. Immunohistochemical study of arginase-1 in the spinal cords of Lewis rats with experimental autoimmune encephalomyelitis. Brain Res. 2012; 1453:77–86.
Article16. Kim J, Choi Y, Ahn M, Jung K, Shin T. Olfactory dysfunction in autoimmune central nervous system neuroinflammation. Mol Neurobiol. 2018; 55:8499–8508.
Article17. Granert C, Raud J, Lindquist L. The polysaccharide fucoidin inhibits the antibiotic-induced inflammatory cascade in experimental pneumococcal meningitis. Clin Diagn Lab Immunol. 1998; 5:322–324.
Article18. Uhm CS, Kim KB, Lim JH, Pee DH, Kim YH, Kim H, Eun BL, Tockgo YC. Effective treatment with fucoidin for perinatal hypoxic-ischemic encephalopathy in rats. Neurosci Lett. 2003; 353:21–24.
Article19. Mecha M, Feliu A, Machín I, Cordero C, Carrillo-Salinas F, Mestre L, Hernández-Torres G, Ortega-Gutiérrez S, López-Rodríguez ML, de Castro F, Clemente D, Guaza C. 2-AG limits Theiler’s virus induced acute neuroinflammation by modulating microglia and promoting MDSCs. Glia. 2018; 66:1447–1463.
Article20. Li JJ, Liu SJ, Liu XY, Ling EA. Herbal compounds with special reference to gastrodin as potential therapeutic agents for microglia mediated neuroin fl ammation. Curr Med Chem. 2018; 02. 14. [Epub]. DOI: 10.2174/0929867325666180214123929.21. Carrillo-Salinas FJ, Mestre L, Mecha M, Feliú A, Del Campo R, Villarrubia N, Espejo C, Montalbán X, Álvarez-Cermeño JC, Villar LM, Guaza C. Gut dysbiosis and neuroimmune responses to brain infection with Theiler's murine encephalomyelitis virus. Sci Rep. 2017; 7:44377.
Article22. Ahn M, Kang J, Lee Y, Riu K, Kim Y, Jee Y, Matsumoto Y, Shin T. Pertussis toxin-induced hyperacute autoimmune encephalomyelitis in Lewis rats is correlated with increased expression of inducible nitric oxide synthase and tumor necrosis factor alpha. Neurosci Lett. 2001; 308:41–44.
Article23. Moon C, Ahn M, Jee Y, Heo S, Kim S, Kim H, Sim KB, Koh CS, Shin YG, Shin T. Sodium salicylate-induced amelioration of experimental autoimmune encephalomyelitis in Lewis rats is associated with the suppression of inducible nitric oxide synthase and cyclooxygenases. Neurosci Lett. 2004; 356:123–126.
Article24. Moon C, Ahn M, Wie MB, Kim HM, Koh CS, Hong SC, Kim MD, Tanuma N, Matsumoto Y, Shin T. Phenidone, a dual inhibitor of cyclooxygenases and lipoxygenases, ameliorates rat paralysis in experimental autoimmune encephalomyelitis by suppressing its target enzymes. Brain Res. 2005; 1035:206–210.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erythropoietin and autoimmune neuroinflammation: lessons from experimental autoimmune encephalomyelitis and experimental autoimmune neuritis
- A Case of Relapsed Acute Disseminated Encephalomyelitis
- Effects of Dexamethasone on Neurogenic Bladder in Experimental Autoimmune Encephalomyelitis Rat
- Protective effect of ethyl acetate extract of Ishige okamurae against carbon tetrachloride-induced acute liver injury in rats
- Mechanism of experimental autoimmune encephalomyelitis in Lewis rats: recent insights from macrophages