Korean J Physiol Pharmacol.
2018 Nov;22(6):679-688. 10.4196/kjpp.2018.22.6.679.
Dual mechanisms for the regulation of brain-derived neurotrophic factor by valproic acid in neural progenitor cells
- Affiliations
-
- 1Department of Life Science, College of Science and Technology, Woosuk University, Jincheon 27841, Korea.
- 2Department of Pharmacology, College of Pharmacy, Chung-Ang University, Seoul 06974, Korea. sunghoonlee@cau.ac.kr
- 3College of Pharmacy, Chung-Ang University, Seoul 06974, Korea.
- 4Department of Pharmaceutical Engineering, College of Life and Health Science, Hoseo University, Asan 31499, Korea.
- 5Department of Medicine, Research Institute of Medical Science, Konkuk University School of Medicine, Chungju 27478, Korea.
- 6Department of Biomedical Science & Technology, Konkuk University, Seoul 05029, Korea.
- 7Department of Pharmacology and Advanced Translational Medicine, School of Medicine, Konkuk University, Seoul 05029, Korea. chanyshin@kku.ac.kr
- KMID: 2430090
- DOI: http://doi.org/10.4196/kjpp.2018.22.6.679
Abstract
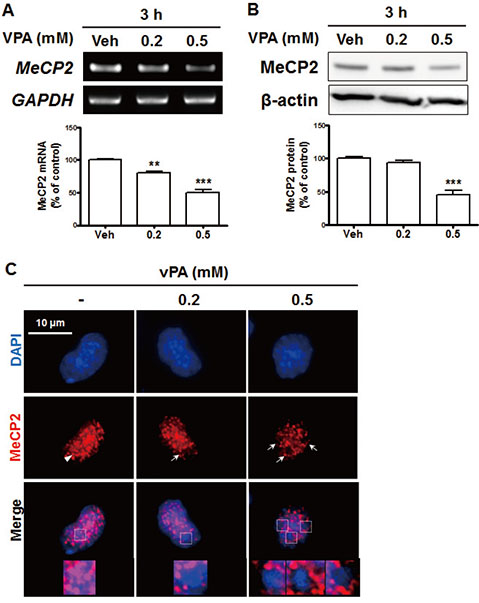
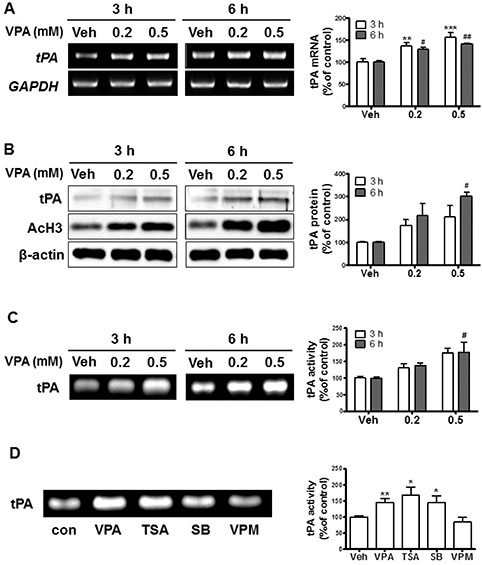
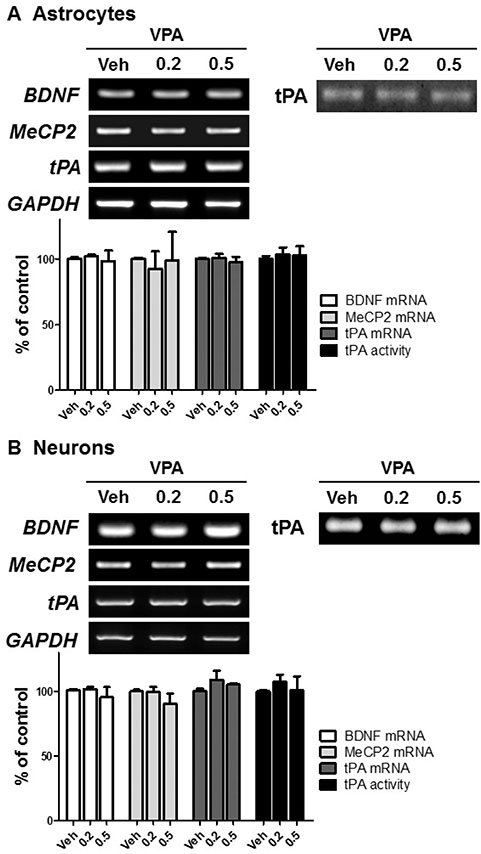
- Autism spectrum disorders (ASDs) are neurodevelopmental disorders that share behavioral features, the results of numerous studies have suggested that the underlying causes of ASDs are multifactorial. Behavioral and/or neurobiological analyses of ASDs have been performed extensively using a valid model of prenatal exposure to valproic acid (VPA). Abnormal synapse formation resulting from altered neurite outgrowth in neural progenitor cells (NPCs) during embryonic brain development has been observed in both the VPA model and ASD subjects. Although several mechanisms have been suggested, the actual mechanism underlying enhanced neurite outgrowth remains unclear. In this study, we found that VPA enhanced the expression of brain-derived neurotrophic factor (BDNF), particularly mature BDNF (mBDNF), through dual mechanisms. VPA increased the mRNA and protein expression of BDNF by suppressing the nuclear expression of methyl-CpG-binding protein 2 (MeCP2), which is a transcriptional repressor of BDNF. In addition, VPA promoted the expression and activity of the tissue plasminogen activator (tPA), which induces BDNF maturation through proteolytic cleavage. Trichostatin A and sodium butyrate also enhanced tPA activity, but tPA activity was not induced by valpromide, which is a VPA analog that does not induce histone acetylation, indicating that histone acetylation activity was required for tPA regulation. VPA-mediated regulation of BDNF, MeCP2, and tPA was not observed in astrocytes or neurons. Therefore, these results suggested that VPA-induced mBDNF upregulation was associated with the dysregulation of MeCP2 and tPA in developing cortical NPCs.
Keyword
MeSH Terms
-
Acetylation
Astrocytes
Autism Spectrum Disorder
Brain
Brain-Derived Neurotrophic Factor*
Butyric Acid
Histones
Methyl-CpG-Binding Protein 2
Neurites
Neurodevelopmental Disorders
Neurons
RNA, Messenger
Stem Cells*
Synapses
Tissue Plasminogen Activator
Up-Regulation
Valproic Acid*
Brain-Derived Neurotrophic Factor
Butyric Acid
Histones
Methyl-CpG-Binding Protein 2
RNA, Messenger
Tissue Plasminogen Activator
Valproic Acid
Figure
Reference
-
1. Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007; 17:103–111.
Article2. Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci. 2006; 29:349–358.
Article3. Eadie MJ. Antiepileptic drugs as human teratogens. Expert Opin Drug Saf. 2008; 7:195–209.
Article4. Rasalam AD, Hailey H, Williams JH, Moore SJ, Turnpenny PD, Lloyd DJ, Dean JC. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev Med Child Neurol. 2005; 47:551–555.
Article5. Schneider T, Przewłocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology. 2005; 30:80–89.
Article6. Schneider T, Turczak J, Przewłocki R. Environmental enrichment reverses behavioral alterations in rats prenatally exposed to valproic acid: issues for a therapeutic approach in autism. Neuropsychopharmacology. 2006; 31:36–46.
Article7. Roullet FI, Lai JK, Foster JA. In utero exposure to valproic acid and autism--a current review of clinical and animal studies. Neurotoxicol Teratol. 2013; 36:47–56.8. Gao R, Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr Mol Med. 2015; 15:146–167.
Article9. Correia CT, Coutinho AM, Sequeira AF, Sousa IG, Lourenco Venda L, Almeida JP, Abreu RL, Lobo C, Miguel TS, Conroy J, Cochrane L, Gallagher L, Gill M, Ennis S, Oliveira GG, Vicente AM. Increased BDNF levels and NTRK2 gene association suggest a disruption of BDNF/TrkB signaling in autism. Genes Brain Behav. 2010; 9:841–848.
Article10. Connolly AM, Chez M, Streif EM, Keeling RM, Golumbek PT, Kwon JM, Riviello JJ, Robinson RG, Neuman RJ, Deuel RM. Brain-derived neurotrophic factor and autoantibodies to neural antigens in sera of children with autistic spectrum disorders, Landau-Kleffner syndrome, and epilepsy. Biol Psychiatry. 2006; 59:354–363.
Article11. Nelson KB, Grether JK, Croen LA, Dambrosia JM, Dickens BF, Jelliffe LL, Hansen RL, Phillips TM. Neuropeptides and neurotrophins in neonatal blood of children with autism or mental retardation. Ann Neurol. 2001; 49:597–606.
Article12. Miyazaki K, Narita N, Sakuta R, Miyahara T, Naruse H, Okado N, Narita M. Serum neurotrophin concentrations in autism and mental retardation: a pilot study. Brain Dev. 2004; 26:292–295.
Article13. Garcia KL, Yu G, Nicolini C, Michalski B, Garzon DJ, Chiu VS, Tongiorgi E, Szatmari P, Fahnestock M. Altered balance of proteolytic isoforms of pro-brain-derived neurotrophic factor in autism. J Neuropathol Exp Neurol. 2012; 71:289–297.
Article14. Zheng Z, Zhang L, Zhu T, Huang J, Qu Y, Mu D. Peripheral brain-derived neurotrophic factor in autism spectrum disorder: a systematic review and meta-analysis. Sci Rep. 2016; 6:31241.
Article15. Hasan MR, Kim JH, Kim YJ, Kwon KJ, Shin CY, Kim HY, Han SH, Choi DH, Lee J. Effect of HDAC inhibitors on neuroprotection and neurite outgrowth in primary rat cortical neurons following ischemic insult. Neurochem Res. 2013; 38:1921–1934.
Article16. Almeida LE, Roby CD, Krueger BK. Increased BDNF expression in fetal brain in the valproic acid model of autism. Mol Cell Neurosci. 2014; 59:57–62.
Article17. Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol. 2001; 63:71–124.
Article18. Nishino S, Ohtomo K, Numata Y, Sato T, Nakahata N, Kurita M. Divergent effects of lithium and sodium valproate on brain-derived neurotrophic factor (BDNF) production in human astrocytoma cells at therapeutic concentrations. Prog Neuropsychopharmacol Biol Psychiatry. 2012; 39:17–22.
Article19. Tsai SJ. Is autism caused by early hyperactivity of brain-derived neurotrophic factor. Med Hypotheses. 2005; 65:79–82.
Article20. Murgatroyd C, Wu Y, Bockmuhl Y, Spengler D. Genes learn from stress: how infantile trauma programs us for depression. Epigenetics. 2010; 5:194–199.
Article21. Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006; 49:341–348.
Article22. Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008; 320:1224–1229.
Article23. Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003; 302:890–893.
Article24. Li W, Pozzo-Miller L. BDNF deregulation in Rett syndrome. Neuropharmacology. 2014; 76 Pt C:737–746.
Article25. Mandel AL, Ozdener H, Utermohlen V. Identification of pro- and mature brain-derived neurotrophic factor in human saliva. Arch Oral Biol. 2009; 54:689–695.
Article26. Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004; 306:487–491.
Article27. Larsson P, Ulfhammer E, Magnusson M, Bergh N, Lunke S, El-Osta A, Medcalf RL, Svensson PA, Karlsson L, Jern S. Role of histone acetylation in the stimulatory effect of valproic acid on vascular endothelial tissue-type plasminogen activator expression. PLoS One. 2012; 7:e31573.
Article28. Lu L, Zhou H, Pan B, Li X, Fu Z, Liu J, Shi Z, Chu T, Wei Z, Ning G, Feng S. c-Jun amino-terminal kinase is involved in valproic acid-mediated neuronal differentiation of mouse embryonic NSCs and neurite outgrowth of NSC-derived neurons. Neurochem Res. 2017; 42:1254–1266.
Article29. Oikawa H, Goh WW, Lim VK, Wong L, Sng JC. Valproic acid mediates miR-124 to down-regulate a novel protein target, GNAI1. Neurochem Int. 2015; 91:62–71.
Article30. Park SW, Lee JG, Seo MK, Cho HY, Lee CH, Lee JH, Lee BJ, Baek JH, Seol W, Kim YH. Effects of mood-stabilizing drugs on dendritic outgrowth and synaptic protein levels in primary hippocampal neurons. Bipolar Disord. 2015; 17:278–290.
Article31. Roullet FI, Wollaston L, Decatanzaro D, Foster JA. Behavioral and molecular changes in the mouse in response to prenatal exposure to the anti-epileptic drug valproic acid. Neuroscience. 2010; 170:514–522.
Article32. Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007; 14:268–276.
Article33. Boulle F, van den Hove DL, Jakob SB, Rutten BP, Hamon M, van Os J, Lesch KP, Lanfumey L, Steinbusch HW, Kenis G. Epigenetic regulation of the BDNF gene: implications for psychiatric disorders. Mol Psychiatry. 2012; 17:584–596.
Article34. Klose R, Bird A. Molecular biology. MeCP2 repression goes nonglobal. Science. 2003; 302:793–795.
Article35. Im HI, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci. 2010; 13:1120–1127.
Article36. Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001; 276:36734–36741.
Article37. Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000; 403:41–45.
Article38. Wu X, Chen PS, Dallas S, Wilson B, Block ML, Wang CC, Kinyamu H, Lu N, Gao X, Leng Y, Chuang DM, Zhang W, Lu RB, Hong JS. Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int J Neuropsychopharmacol. 2008; 11:1123–1134.
Article39. Wei R, Lin CM, Tu YY. Strain-specific BDNF expression of rat primary astrocytes. J Neuroimmunol. 2010; 220:90–98.
Article40. Harrison IF, Crum WR, Vernon AC, Dexter DT. Neurorestoration induced by the HDAC inhibitor sodium valproate in the lactacystin model of Parkinson's is associated with histone acetylation and upregulation of neurotrophic factors. Br J Pharmacol. 2015; 172:4200–4215.
Article41. Croce N, Mathe AA, Gelfo F, Caltagirone C, Bernardini S, Angelucci F. Effects of lithium and valproic acid on BDNF protein and gene expression in an in vitro human neuron-like model of degeneration. J Psychopharmacol. 2014; 28:964–972.
Article42. Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990; 5:501–509.
Article43. Fukumitsu H, Ohtsuka M, Murai R, Nakamura H, Itoh K, Furukawa S. Brain-derived neurotrophic factor participates in determination of neuronal laminar fate in the developing mouse cerebral cortex. J Neurosci. 2006; 26:13218–13230.
Article44. Ben-Ari Y. Neuro-archaeology: pre-symptomatic architecture and signature of neurological disorders. Trends Neurosci. 2008; 31:626–636.
Article45. Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry. 2009; 14:51–59.
Article46. Chen PS, Peng GS, Li G, Yang S, Wu X, Wang CC, Wilson B, Lu RB, Gean PW, Chuang DM, Hong JS. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol Psychiatry. 2006; 11:1116–1125.
Article47. Girdhar K, Hoffman GE, Jiang Y, Brown L, Kundakovic M, Hauberg ME, Francoeur NJ, Wang YC, Shah H, Kavanagh DH, Zharovsky E, Jacobov R, Wiseman JR, Park R, Johnson JS, Kassim BS, Sloofman L, Mattei E, Weng Z, Sieberts SK, et al. Cell-specific histone modification maps in the human frontal lobe link schizophrenia risk to the neuronal epigenome. Nat Neurosci. 2018; 21:1126–1136.
Article48. Lee JH, Hart SR, Skalnik DG. Histone deacetylase activity is required for embryonic stem cell differentiation. Genesis. 2004; 38:32–38.
Article49. Cho B, Kim HJ, Kim H, Sun W. Changes in the histone acetylation patterns during the development of the nervous system. Exp Neurobiol. 2011; 20:81–84.
Article50. Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002; 328:261–264.
Article51. Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998; 37:1553–1561.
Article52. Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, Knudsen GM, Aznar S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2011; 14:347–353.
Article53. Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008; 64:527–532.
Article54. Chen SL, Lee SY, Chang YH, Chen PS, Lee IH, Wang TY, Chen KC, Yang YK, Hong JS, Lu RB. Therapeutic effects of add-on low-dose dextromethorphan plus valproic acid in bipolar disorder. Eur Neuropsychopharmacol. 2014; 24:1753–1759.
Article55. Cunha AB, Frey BN, Andreazza AC, Goi JD, Rosa AR, Goncalves CA, Santin A, Kapczinski F. Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. Neurosci Lett. 2006; 398:215–219.
Article56. Yoshimura R, Mitoma M, Sugita A, Hori H, Okamoto T, Umene W, Ueda N, Nakamura J. Effects of paroxetine or milnacipran on serum brain-derived neurotrophic factor in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2007; 31:1034–1037.
Article57. Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S, Iyo M. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003; 54:70–75.
Article58. Dias VV, Brissos S, Frey BN, Andreazza AC, Cardoso C, Kapczinski F. Cognitive function and serum levels of brain-derived neurotrophic factor in patients with bipolar disorder. Bipolar Disord. 2009; 11:663–671.
Article59. Piccinni A, Marazziti D, Catena M, Domenici L, Del Debbio A, Bianchi C, Mannari C, Martini C, Da Pozzo E, Schiavi E, Mariotti A, Roncaglia I, Palla A, Consoli G, Giovannini L, Massimetti G, Dell'Osso L. Plasma and serum brain-derived neurotrophic factor (BDNF) in depressed patients during 1 year of antidepressant treatments. J Affect Disord. 2008; 105:279–283.60. Hellweg R, Ziegenhorn A, Heuser I, Deuschle M. Serum concentrations of nerve growth factor and brain-derived neurotrophic factor in depressed patients before and after antidepressant treatment. Pharmacopsychiatry. 2008; 41:66–71.
Article61. Calfa G, Percy AK, Pozzo-Miller L. Experimental models of Rett syndrome based on Mecp2 dysfunction. Exp Biol Med (Maywood). 2011; 236:3–19.
Article62. Li W, Pozzo-Miller L. Beyond widespread Mecp2 deletions to model rett syndrome: conditional spatio-temporal knockout, single-point mutations and transgenic rescue mice. Autism Open Access. 2012; 2012:5.
Article63. Nguyen MV, Du F, Felice CA, Shan X, Nigam A, Mandel G, Robinson JK, Ballas N. MeCP2 is critical for maintaining mature neuronal networks and global brain anatomy during late stages of postnatal brain development and in the mature adult brain. J Neurosci. 2012; 32:10021–10034.
Article64. Larimore JL, Chapleau CA, Kudo S, Theibert A, Percy AK, Pozzo-Miller L. Bdnf overexpression in hippocampal neurons prevents dendritic atrophy caused by Rett-associated MECP2 mutations. Neurobiol Dis. 2009; 34:199–211.
Article65. Klose RJ, Sarraf SA, Schmiedeberg L, McDermott SM, Stancheva I, Bird AP. DNA binding selectivity of MeCP2 due to a requirement for A/T sequences adjacent to methyl-CpG. Mol Cell. 2005; 19:667–678.
Article66. Sun AX, Crabtree GR, Yoo AS. MicroRNAs: regulators of neuronal fate. Curr Opin Cell Biol. 2013; 25:215–221.
Article67. Volvert ML, Rogister F, Moonen G, Malgrange B, Nguyen L. MicroRNAs tune cerebral cortical neurogenesis. Cell Death Differ. 2012; 19:1573–1581.
Article68. Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011; 12:861–874.
Article69. Im HI, Kenny PJ. MicroRNAs in neuronal function and dysfunction. Trends Neurosci. 2012; 35:325–334.
Article70. Geaghan M, Cairns MJ. MicroRNA and posttranscriptional dysregulation in psychiatry. Biol Psychiatry. 2015; 78:231–239.
Article71. Hara Y, Ago Y, Takano E, Hasebe S, Nakazawa T, Hashimoto H, Matsuda T, Takuma K. Prenatal exposure to valproic acid increases miR-132 levels in the mouse embryonic brain. Mol Autism. 2017; 8:33.
Article72. Klein ME, Lioy DT, Ma L, Impey S, Mandel G, Goodman RH. Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat Neurosci. 2007; 10:1513–1514.
Article73. Good KV, Martinez de, Tyagi M, Cheema MS, Thambirajah AA, Gretzinger TL, Stefanelli G, Chow RL, Krupke O, Hendzel M, Missiaen K, Underhill A, Landsberger N, Ausio J. Trichostatin A decreases the levels of MeCP2 expression and phosphorylation and increases its chromatin binding affinity. Epigenetics. 2017; 12:934–944.
Article74. Larsson P, Alwis I, Niego B, Sashindranath M, Fogelstrand P, Wu MC, Glise L, Magnusson M, Daglas M, Bergh N, Jackson SP, Medcalf RL, Jern S. Valproic acid selectively increases vascular endothelial tissue-type plasminogen activator production and reduces thrombus formation in the mouse. J Thromb Haemost. 2016; 14:2496–2508.
Article75. Olesen JB, Hansen PR, Abildstrøm SZ, Andersson C, Weeke P, Schmiegelow M, Erdal J, Torp-Pedersen C, Gislason GH. Valproate attenuates the risk of myocardial infarction in patients with epilepsy: a nationwide cohort study. Pharmacoepidemiol Drug Saf. 2011; 20:146–153.
Article76. Dregan A, Charlton J, Wolfe CD, Gulliford MC, Markus HS. Is sodium valproate, an HDAC inhibitor, associated with reduced risk of stroke and myocardial infarction? A nested case-control study. Pharmacoepidemiol Drug Saf. 2014; 23:759–767.
Article77. Kooistra T, van den, Tons A, Platenburg G, Rijken DC, van den Berg J. Butyrate stimulates tissue-type plasminogen-activator synthesis in cultured human endothelial cells. Biochem J. 1987; 247:605–612.
Article78. Arts J, Lansink M, Grimbergen J, Toet KH, Kooistra T. Stimulation of tissue-type plasminogen activator gene expression by sodium butyrate and trichostatin A in human endothelial cells involves histone acetylation. Biochem J. 1995; 310:171–176.
Article79. Dunoyer-Geindre S, Kruithof EK. Epigenetic control of tissue-type plasminogen activator synthesis in human endothelial cells. Cardiovasc Res. 2011; 90:457–463.
Article80. Zhang Y, Dufau ML. Silencing of transcription of the human luteinizing hormone receptor gene by histone deacetylase-mSin3A complex. J Biol Chem. 2002; 277:33431–33438.
Article81. Kim S, Kang JK, Kim YK, Seo DW, Ahn SH, Lee JC, Lee CH, You JS, Cho EJ, Lee HW, Han JW. Histone deacetylase inhibitor apicidin induces cyclin E expression through Sp1 sites. Biochem Biophys Res Commun. 2006; 342:1168–1173.
Article82. Sowa Y, Orita T, Minamikawa S, Nakano K, Mizuno T, Nomura H, Sakai T. Histone deacetylase inhibitor activates the WAF1/Cip1 gene promoter through the Sp1 sites. Biochem Biophys Res Commun. 1997; 241:142–150.
Article83. Arts J, Herr I, Lansink M, Angel P, Kooistra T. Cell-type specific DNA-protein interactions at the tissue-type plasminogen activator promoter in human endothelial and HeLa cells in vivo and in vitro. Nucleic Acids Res. 1997; 25:311–317.
Article84. Medcalf RL, Ruegg M, Schleuning WD. A DNA motif related to the cAMP-responsive element and an exon-located activator protein-2 binding site in the human tissue-type plasminogen activator gene promoter cooperate in basal expression and convey activation by phorbol ester and cAMP. J Biol Chem. 1990; 265:14618–14626.
Article85. Lee SH, Ko HM, Kwon KJ, Lee J, Han SH, Han DW, Cheong JH, Ryu JH, Shin CY. tPA regulates neurite outgrowth by phosphorylation of LRP5/6 in neural progenitor cells. Mol Neurobiol. 2014; 49:199–215.
Article86. Cho KS, Kwon KJ, Choi CS, Jeon SJ, Kim KC, Park JH, Ko HM, Lee SH, Cheong JH, Ryu JH, Han SH, Shin CY. Valproic acid induces astrocyte-dependent neurite outgrowth from cultured rat primary cortical neuron via modulation of tPA/PAI-1 activity. Glia. 2013; 61:694–709.
Article87. Gravanis I, Tsirka SE. Tissue plasminogen activator and glial function. Glia. 2005; 49:177–183.
Article88. Hastings GA, Coleman TA, Haudenschild CC, Stefansson S, Smith EP, Barthlow R, Cherry S, Sandkvist M, Lawrence DA. Neuroserpin, a brain-associated inhibitor of tissue plasminogen activator is localized primarily in neurons. Implications for the regulation of motor learning and neuronal survival. J Biol Chem. 1997; 272:33062–33067.89. Berg JM, Geschwind DH. Autism genetics: searching for specificity and convergence. Genome Biol. 2012; 13:247.
Article90. State MW, Levitt P. The conundrums of understanding genetic risks for autism spectrum disorders. Nat Neurosci. 2011; 14:1499–1506.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epigenetically Upregulated T-Type Calcium Channels Contribute to Abnormal Proliferation of Embryonic Neural Progenitor Cells Exposed to Valproic Acid
- In Vitro Neural Cell Differentiation Derived from Human Embryonic Stem Cells: Effects of PDGF-bb and BDNF on the Generation of Functional Neurons
- Expression of mRNAs for Neurotrophic Factors in Human Neural Stem Cells Derived from Fetal Telencephalon
- The New Neurobiology of Depression
- Increased Expression of Brain-derived Neurotrophic Factor in Irritable Bowel Syndrome and Its Correlation With Abdominal Pain (Gut 2012;61:685-694)