Korean J Radiol.
2019 Jan;20(1):69-82. 10.3348/kjr.2018.0231.
Introduction of a New Staging System of Breast Cancer for Radiologists: An Emphasis on the Prognostic Stage
- Affiliations
-
- 1Department of Radiology, Severance Hospital, Research Institute of Radiological Science, Yonsei University College of Medicine, Seoul, Korea. mines@yuhs.ac
- 2Department of Radiology, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- KMID: 2429921
- DOI: http://doi.org/10.3348/kjr.2018.0231
Abstract
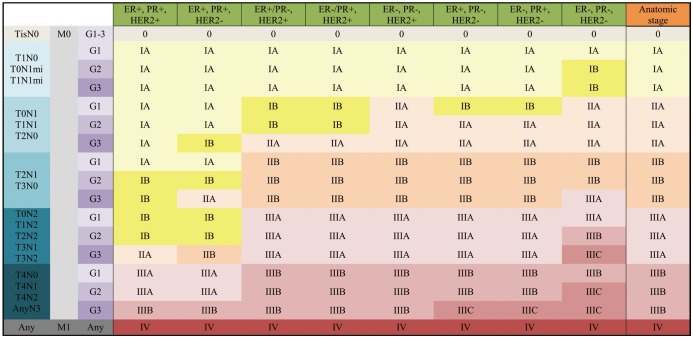
- In 2017, the American Joint Committee on Cancer announced the 8th edition of its cancer staging system. For breast cancer, the most significant change in the staging system is the incorporation of biomarkers into the anatomic staging to create prognostic stages. Different prognostic stages are assigned to tumors with the same anatomic stages according to the tumor grade, hormone receptor (estrogen receptor; progesterone receptor) status, and HER2 status. A Clinical Prognostic Stage is assigned to all patients regardless of the type of therapy used; in contrast, a Pathologic Prognosis Stage is assigned to patients in whom surgery is the initial treatment. In a few situations, low Oncotype DX recurrence scores can change the prognostic stage. The radiologists need to understand the importance of the biologic factors that can influence cancer staging.
Keyword
MeSH Terms
Figure
Reference
-
1. Gabriel NH, James LC, Carl JD, Stephen BE, Elizabeth AM, Hope SR, et al. Breast. In : Mahul BA, editor. American Joint Committee on Cancer (AJCC). AJCC cancer staging manual. 8th ed. New York, NY: Springer;2017. p. 589–628.2. Prat A, Pineda E, Adamo B, Galván P, Fernández A, Gaba L, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015; 24(Suppl 2):S26–S35. PMID: 26253814.
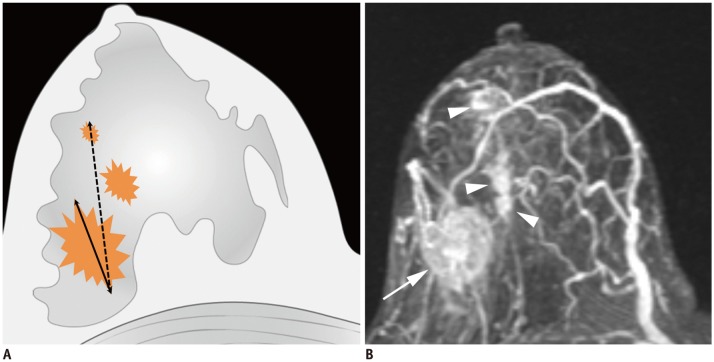
Article3. Rezo A, Dahlstrom J, Shadbolt B, Rodins K, Zhang Y, Davis AJ. ACT & SENSW BCTG. Tumor size and survival in multicentric and multifocal breast cancer. Breast. 2011; 20:259–263. PMID: 21324695.
Article4. Morris EA, Schwartz LH, Drotman MB, Kim SJ, Tan LK, Liberman L, et al. Evaluation of pectoralis major muscle in patients with posterior breast tumors on breast MR images: early experience. Radiology. 2000; 214:67–72. PMID: 10644103.
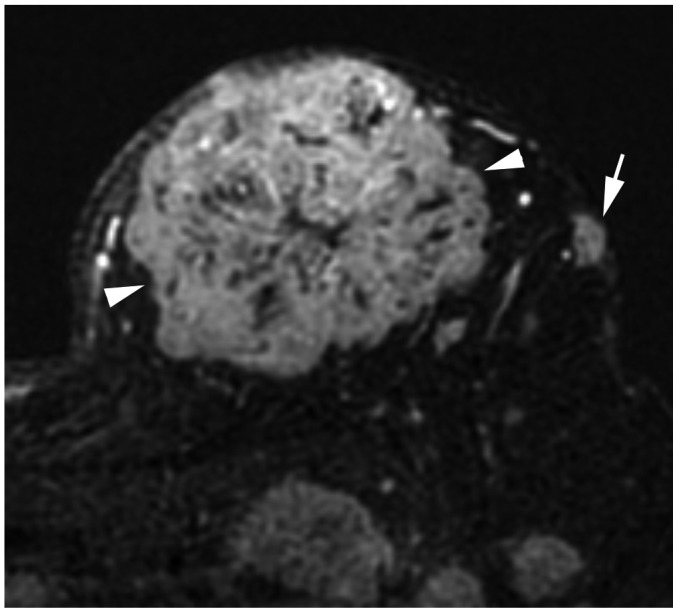
Article5. Lee EJ, Han SH, Kang BJ, Kim SH. Imaging and pathologic characterization of the skin thickening or enhancement under the breast MRI. Investig Magn Reson Imaging. 2016; 20:9–26.
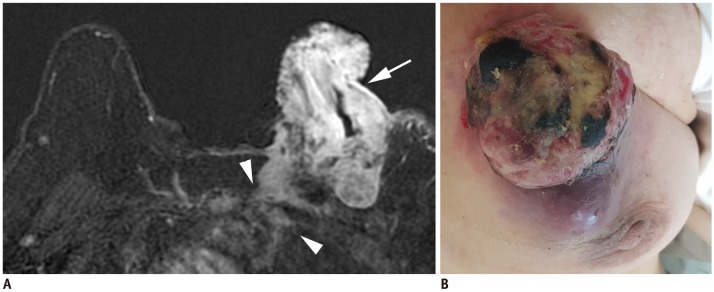
Article6. Chang S, Parker SL, Pham T, Buzdar AU, Hursting SD. Inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program of the National Cancer Institute, 1975–1992. Cancer. 1998; 82:2366–2372. PMID: 9635529.7. Levine PH, Steinhorn SC, Ries LG, Aron JL. Inflammatory breast cancer: the experience of the surveillance, epidemiology, and end results (SEER) program. J Natl Cancer Inst. 1985; 74:291–297. PMID: 3856043.8. Yang WT, Le-Petross HT, Macapinlac H, Carkaci S, Gonzalez-Angulo AM, Dawood S, et al. Inflammatory breast cancer: PET/CT, MRI, mammography, and sonography findings. Breast Cancer Res Treat. 2008; 109:417–426. PMID: 17653852.
Article9. Le-Petross HT, Cristofanilli M, Carkaci S, Krishnamurthy S, Jackson EF, Harrell RK, et al. MRI features of inflammatory breast cancer. AJR Am J Roentgenol. 2011; 197:W769–W776. PMID: 21940550.
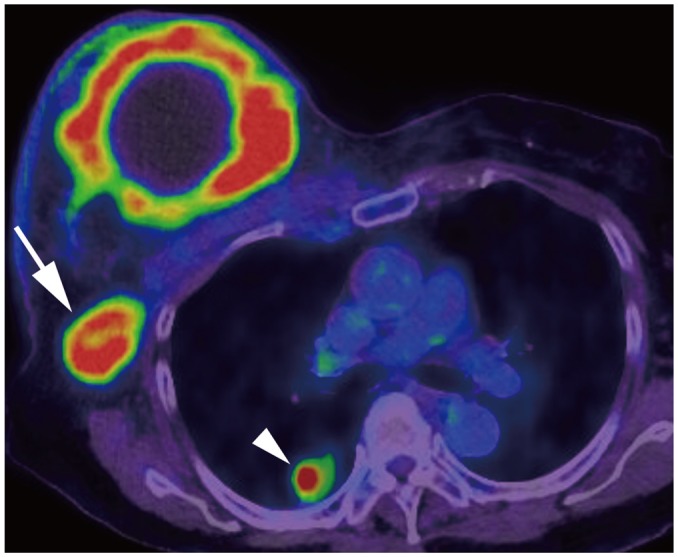
Article10. Alvarez S, Añorbe E, Alcorta P, López F, Alonso I, Cortés J. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol. 2006; 186:1342–1348. PMID: 16632729.
Article11. Patel T, Given-Wilson RM, Thomas V. The clinical importance of axillary lymphadenopathy detected on screening mammography: revisited. Clin Radiol. 2005; 60:64–71. PMID: 15642295.
Article12. Mortellaro VE, Marshall J, Singer L, Hochwald SN, Chang M, Copeland EM, et al. Magnetic resonance imaging for axillary staging in patients with breast cancer. J Magn Reson Imaging. 2009; 30:309–312. PMID: 19466713.
Article13. Valente SA, Levine GM, Silverstein MJ, Rayhanabad JA, Weng-Grumley JG, Ji L, et al. Accuracy of predicting axillary lymph node positivity by physical examination, mammography, ultrasonography, and magnetic resonance imaging. Ann Surg Oncol. 2012; 19:1825–1830. PMID: 22227922.
Article14. Yoshimura G, Sakurai T, Oura S, Suzuma T, Tamaki T, Umemura T, et al. Evaluation of axillary lymph node status in breast cancer with MRI. Breast Cancer. 1999; 6:249–258. PMID: 11091725.
Article15. Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010; 252:426–432. discussion 432-433. PMID: 20739842.16. Humphrey KL, Saksena MA, Freer PE, Smith BL, Rafferty EA. To do or not to do: axillary nodal evaluation after ACOSOG Z0011 trial. Radiographics. 2014; 34:1807–1816. PMID: 25384280.
Article17. Verheuvel NC, van den Hoven I, Ooms HW, Voogd AC, Roumen RM. The role of ultrasound-guided lymph node biopsy in axillary staging of invasive breast cancer in the post-ACOSOG Z0011 trial era. Ann Surg Oncol. 2015; 22:409–415. PMID: 25205303.
Article18. Farrell TP, Adams NC, Stenson M, Carroll PA, Griffin M, Connolly EM, et al. The Z0011 trial: is this the end of axillary ultrasound in the pre-operative assessment of breast cancer patients? Eur Radiol. 2015; 25:2682–2687. PMID: 25740803.
Article19. Patanaphan V, Salazar OM, Risco R. Breast cancer: metastatic patterns and their prognosis. South Med J. 1988; 81:1109–1112. PMID: 3420442.20. Aebi S, Davidson T, Gruber G, Castiglione M. ESMO Guidelines Working Group. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010; 21(Suppl 5):v9–v14. PMID: 20555111.
Article21. Rapoport BL, Demetriou GS, Moodley SD, Benn CA. When and how do I use neoadjuvant chemotherapy for breast cancer? Curr Treat Options Oncol. 2014; 15:86–98. PMID: 24306808.
Article22. Yi M, Mittendorf EA, Cormier JN, Buchholz TA, Bilimoria K, Sahin AA, et al. Novel staging system for predicting disease-specific survival in patients with breast cancer treated with surgery as the first intervention: time to modify the current American Joint Committee on Cancer staging system. J Clin Oncol. 2011; 29:4654–4661. PMID: 22084362.
Article23. Schwartz AM, Henson DE, Chen D, Rajamarthandan S. Histologic grade remains a prognostic factor for breast cancer regardless of the number of positive lymph nodes and tumor size: a study of 161 708 cases of breast cancer from the SEER Program. Arch Pathol Lab Med. 2014; 138:1048–1052. PMID: 25076293.24. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991; 19:403–410. PMID: 1757079.
Article25. Elston EW, Ellis IO. Method for grading breast cancer. J Clin Pathol. 1993; 46:189–190.
Article26. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Davies C, Godwin J, Gray R, Clarke M, Cutter D, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011; 378:771–784. PMID: 21802721.27. Barnes DM, Harris WH, Smith P, Millis RR, Rubens RD. Immunohistochemical determination of oestrogen receptor: comparison of different methods of assessment of staining and correlation with clinical outcome of breast cancer patients. Br J Cancer. 1996; 74:1445–1451. PMID: 8912543.
Article28. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987; 235:177–182. PMID: 3798106.29. Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009; 14:320–368. PMID: 19346299.
Article30. Rosenthal SI, Depowski PL, Sheehan CE, Ross JS. Comparison of HER-2/neu oncogene amplification detected by fluorescence in situ hybridization in lobular and ductal breast cancer. Appl Immunohistochem Mol Morphol. 2002; 10:40–46. PMID: 11893034.31. Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Breast Cancer International Research Group. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011; 365:1273–1283. PMID: 21991949.
Article32. Moasser MM, Krop IE. The evolving landscape of HER2 targeting in breast cancer. JAMA Oncol. 2015; 1:1154–1161. PMID: 26204261.
Article33. Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983; 31:13–20. PMID: 6339421.
Article34. Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, et al. Panel Members. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015; 26:1533–1546. PMID: 25939896.
Article35. Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003; 95:142–153. PMID: 12529347.
Article36. Eiermann W, Rezai M, Kümmel S, Kühn T, Warm M, Friedrichs K, et al. The 21-gene recurrence score assay impacts adjuvant therapy recommendations for ER-positive, node-negative and node-positive early breast cancer resulting in a risk-adapted change in chemotherapy use. Ann Oncol. 2013; 24:618–624. PMID: 23136233.
Article37. Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015; 373:2005–2014. PMID: 26412349.
Article38. Stemmer S, Steiner M, Rizel S, Ben-Baruch N, Soussan-Gutman L, Rosengarten O, et al. 1963 first prospective outcome data in 930 patients with more than 5 year median follow up in whom treatment decisions in clinical practice have been made incorporating the 21-gene recurrence score. European Journal of Cancer. 2015; 51:S321.
Article39. Stemmer S, Steiner M, Rizel S, Soussan-Gutman L, Geffen DB, Nisenbaum B, et al. Abstract P5-08-02: real-life analysis evaluating 1594 N0/Nmic breast cancer patients for whom treatment decisions incorporated the 21-gene recurrence score result: 5-year KM estimate for breast cancer specific survival with recurrence score results ≤30 is >98%. Cancer Research. 2016; 76(4 Suppl):Abstract nr P5-08-02.40. Gluz O, Nitz UA, Christgen M, Kates RE, Shak S, Clemens M, et al. West German Study Group phase III planB trial: first prospective outcome data for the 21-gene recurrence score assay and concordance of prognostic markers by central and local pathology assessment. J Clin Oncol. 2016; 34:2341–2349. PMID: 26926676.
Article41. Petkov VI, Miller DP, Howlader N, Gliner N, Howe W, Schussler N, et al. Breast-cancer-specific mortality in patients treated based on the 21-gene assay: a SEER population-based study. NPJ Breast Cancer. 2016; 2:16017. PMID: 28721379.
Article42. Drukker CA, Bueno-de-Mesquita JM, Retèl VP, van Harten WH, van Tinteren H, Wesseling J, et al. A prospective evaluation of a breast cancer prognosis signature in the observational RASTER study. Int J Cancer. 2013; 133:929–936. PMID: 23371464.
Article43. Filipits M, Rudas M, Jakesz R, Dubsky P, Fitzal F, Singer CF, et al. EP Investigators. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res. 2011; 17:6012–6020. PMID: 21807638.
Article44. Dubsky P, Filipits M, Jakesz R, Rudas M, Singer CF, Greil R, et al. Austrian Breast and Colorectal Cancer Study Group (ABCSG). EndoPredict improves the prognostic classification derived from common clinical guidelines in ER-positive, HER2-negative early breast cancer. Ann Oncol. 2013; 24:640–647. PMID: 23035151.
Article45. Filipits M, Nielsen TO, Rudas M, Greil R, Stöger H, Jakesz R, et al. Austrian Breast and Colorectal Cancer Study Group. The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clin Cancer Res. 2014; 20:1298–1305. PMID: 24520097.
Article46. Sestak I, Cuzick J, Dowsett M, Lopez-Knowles E, Filipits M, Dubsky P, et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol. 2015; 33:916–922. PMID: 25332252.
Article47. Sgroi DC, Sestak I, Cuzick J, Zhang Y, Schnabel CA, Schroeder B, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol. 2013; 14:1067–1076. PMID: 24035531.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Introduction of a New Staging System of Breast Cancer for Radiologists: An Emphasis on the Prognostic Stage
- Assessment of the Prognostic Staging System of American Joint Committee on Cancer 8th Edition for Breast Cancer: Comparisons with the Conventional Anatomic Staging System
- Development of an Excel Program for the Updated Eighth American Joint Committee on Cancer Breast Cancer Staging System
- Updated guidelines on the preoperative staging of thyroid cancer
- Prognostic Validation of the American Joint Committee on Cancer 8th Staging System in 24,014 Korean Patients with Breast Cancer