J Breast Cancer.
2018 Dec;21(4):406-414. 10.4048/jbc.2018.21.e61.
T-Cell Immunoglobulin Mucin 3 Expression on Tumor Infiltrating Lymphocytes as a Positive Prognosticator in Triple-Negative Breast Cancer
- Affiliations
-
- 1Department of Surgery, Dong-A University College of Medicine, Busan, Korea.
- 2Breast Medical Center, Dong-A University College of Medicine, Busan, Korea.
- 3Department of Pathology, Dong-A University College of Medicine, Busan, Korea. jsjung1@dau.ac.kr
- 4Department of Radiology, Dong-A University College of Medicine, Busan, Korea.
- KMID: 2429819
- DOI: http://doi.org/10.4048/jbc.2018.21.e61
Abstract
- PURPOSE
T-cell immunoglobulin and mucin domain-containing molecule 3 (TIM-3) is an emerging immune response molecule related to T-cell anergy. There has been tremendous interest in breast cancer targeting immune checkpoint molecules, especially in the triple-negative breast cancer (TNBC). This study was designed to investigate TIM-3 expression on tumor infiltrating lymphocytes (TILs), its relationships with clinicopathological para-meters and expression of programmed death receptor 1 (PD-1)/programmed death receptor ligand 1 (PD-L1), and its prognostic role.
METHODS
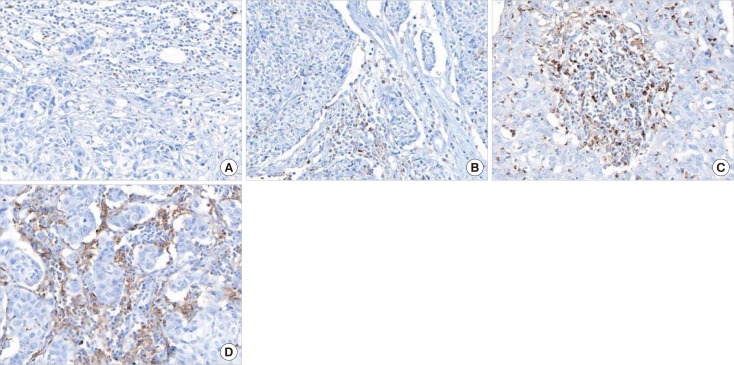
Immunohistochemistry on tissue microarray blocks produced from 109 samples of invasive ductal carcinoma type TNBC was performed with antibodies toward TIM-3, PD-1, PD-L1 and breast cancer-related molecular markers. Associations between their expression and clinicopathological parameters as well as survival analyses were performed.
RESULTS
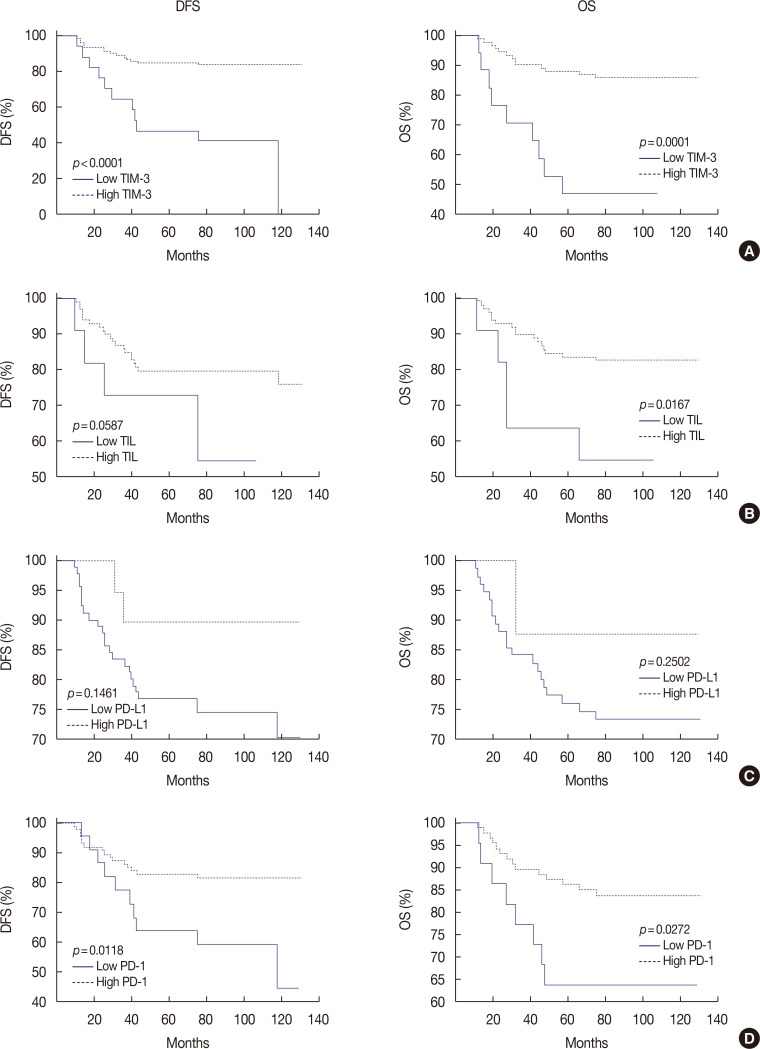
TIM-3 was expressed in TILs from all 109 TNBCs, consisting of 17 cases ( < 5%), 31 cases (6%-25%), 48 cases (26%-50%), and 13 cases (>51%). High TIM-3 was significantly correlated with younger patients (p=0.0101), high TILs (p=0.0029), high tumor stage (p=0.0018), high PD-1 (p=0.0001) and high PD-L1 (p=0.0019), and tended to be associated with higher histologic grade, absence of extensive in situ components and microcalcification. High TIM-3 expression was significantly associated with a combinational immunophenotype group of high PD-L1 and high PD-1 (p < 0.0001). High TIM-3 demonstrated a significantly better disease-free survival (DFS) (p < 0.0001) and longer overall survival (OS) (p=0.0001), together with high TILs and high PD-1. In univariate survival analysis, high TIM-3 showed reduced relapse risk (p < 0.0001) and longer OS (p=0.0003), together with high PD-1 expression. In multivariate analysis, high TIM-3 was statistically significant in predicting prognosis, showing better DFS (hazard ratio [HR], 0.0994; 95% confidence interval [CI], 0.0296-0.3337; p=0.0002) and longer OS (HR, 0.1109; 95% CI, 0.0314-0.3912; p=0.0006).
CONCLUSION
In this study, we demonstrate that TIM-3 expression is an independent positive prognostic factor in TNBC, despite its association with poor clinical and pathologic features.
Keyword
MeSH Terms
Figure
Reference
-
1. Liedtke C, Gonzalez-Angulo AM, Pusztai L. Definition of triple-negative breast cancer and relationship to basal-like molecular subtype. In : DeVita VT, Lawrence TS, Rosenberg SA, editors. DeVita, Hellman, and Rosenberg's Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia: Lippincott Williams & Wilkins;2011. p. 1–6.2. Stovgaard ES, Nielsen D, Hogdall E, Balslev E. Triple negative breast cancer: prognostic role of immune-related factors: a systematic review. Acta Oncol. 2018; 57:74–82. PMID: 29168430.3. Ibrahim EM, Al-Foheidi ME, Al-Mansour MM, Kazkaz GA. The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat. 2014; 148:467–476. PMID: 25361613.
Article4. Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011; 29:1949–1955. PMID: 21483002.
Article5. Matsumoto H, Thike AA, Li H, Yeong J, Koo SL, Dent RA, et al. Increased CD4 and CD8-positive T cell infiltrate signifies good prognosis in a subset of triple-negative breast cancer. Breast Cancer Res Treat. 2016; 156:237–247. PMID: 26960711.
Article6. Pentcheva-Hoang T, Corse E, Allison JP. Negative regulators of T-cell activation: potential targets for therapeutic intervention in cancer, auto-immune disease, and persistent infections. Immunol Rev. 2009; 229:67–87. PMID: 19426215.
Article7. Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007; 7:95–106. PMID: 17251916.
Article8. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; 366:2443–2454. PMID: 22658127.
Article9. Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010; 235:172–189. PMID: 20536563.10. Wu J, Lin G, Zhu Y, Zhang H, Shi G, Shen Y, et al. Low TIM3 expression indicates poor prognosis of metastatic prostate cancer and acts as an independent predictor of castration resistant status. Sci Rep. 2017; 7:8869. PMID: 28827755.
Article11. Yu M, Lu B, Liu Y, Me Y, Wang L, Zhang P. Tim-3 is upregulated in human colorectal carcinoma and associated with tumor progression. Mol Med Rep. 2017; 15:689–695. PMID: 28035413.
Article12. Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005; 6:1245–1252. PMID: 16286920.
Article13. Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010; 207:2175–2186. PMID: 20819923.
Article14. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015; 26:259–271. PMID: 25214542.
Article15. Bubendorf L, Nocito A, Moch H, Sauter G. Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. J Pathol. 2001; 195:72–79. PMID: 11568893.16. Soo RA, Kim HR, Asuncion BR, Fazreen Z, Omar MF, Herrera MC, et al. Significance of immune checkpoint proteins in EGFR-mutant non-small cell lung cancer. Lung Cancer. 2017; 105:17–22. PMID: 28236980.17. Muenst S, Schaerli AR, Gao F, Däster S, Trella E, Droeser RA, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014; 146:15–24. PMID: 24842267.
Article18. Jang SH, Lee JE, Oh MH, Lee JH, Cho HD, Kim KJ, et al. High EZH2 protein expression is associated with poor overall survival in patients with luminal A breast cancer. J Breast Cancer. 2016; 19:53–60. PMID: 27066096.
Article19. Zhang H, Xiang R, Wu B, Li J, Luo G. T-cell immunoglobulin mucin-3 expression in invasive ductal breast carcinoma: clinicopathological correlations and association with tumor infiltration by cytotoxic lymphocytes. Mol Clin Oncol. 2017; 7:557–563. PMID: 28855989.
Article20. Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, et al. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS One. 2012; 7:e30676. PMID: 22363469.
Article21. Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012; 56:1342–1351. PMID: 22505239.
Article22. Liu JF, Ma SR, Mao L, Bu LL, Yu GT, Li YC, et al. T-cell immunoglobulin mucin 3 blockade drives an antitumor immune response in head and neck cancer. Mol Oncol. 2017; 11:235–247. PMID: 28102051.
Article23. Ghebeh H, Barhoush E, Tulbah A, Elkum N, Al-Tweigeri T, Dermime S. FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: implication for immunotherapy. BMC Cancer. 2008; 8:57. PMID: 18294387.
Article24. Bae SB, Cho HD, Oh MH, Lee JH, Jang SH, Hong SA, et al. Expression of programmed death receptor ligand 1 with high tumor-infiltrating lymphocytes is associated with better prognosis in breast cancer. J Breast Cancer. 2016; 19:242–251. PMID: 27721873.
Article25. Baptista MZ, Sarian LO, Derchain SF, Pinto GA, Vassallo J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol. 2016; 47:78–84. PMID: 26541326.
Article26. Tsang JY, Au WL, Lo KY, Ni YB, Hlaing T, Hu J, et al. PD-L1 expression and tumor infiltrating PD-1+ lymphocytes associated with outcome in HER2+ breast cancer patients. Breast Cancer Res Treat. 2017; 162:19–30. PMID: 28058578.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Issues and Clinical Evidence in Tumor-Infiltrating Lymphocytes in Breast Cancer
- Association between p53 Expression and Amount of Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer
- Prognostic Role and Clinical Association of Tumor-Infiltrating Lymphocyte, Programmed Death Ligand-1 Expression with Neutrophil-Lymphocyte Ratio in Locally Advanced Triple-Negative Breast Cancer
- Overexpression of Cell Cycle Progression Inhibitor Geminin is Associated with Tumor Stem-Like Phenotype of Triple-Negative Breast Cancer
- Prognostic Influence of BCL2 on Molecular Subtypes of Breast Cancer