Nutr Res Pract.
2018 Feb;12(1):29-40. 10.4162/nrp.2018.12.1.29.
Topical or oral treatment of peach flower extract attenuates UV-induced epidermal thickening, matrix metalloproteinase-13 expression and pro-inflammatory cytokine production in hairless mice skin
- Affiliations
-
- 1Institute on Aging, Seoul National University College of Medicine, #304 Biomedical building, 103 Daehak-ro, Jongno-gu, Seoul 03080, Korea. kwakcs@snu.ac.kr
- 2Institute of Human-Environment Interface Biology, Seoul National University College of Medicine, Seoul 03080, Korea.
- 3Department of Dermatology, Seoul National University College of Medicine, Seoul 03080, Korea.
- 4Laboratoy of Cutaneous Aging Research, Biomedical Research Institute, Seoul National University Hospital, Seoul 03080, Korea.
- KMID: 2429016
- DOI: http://doi.org/10.4162/nrp.2018.12.1.29
Abstract
- BACKGROUND/OBJECTIVES
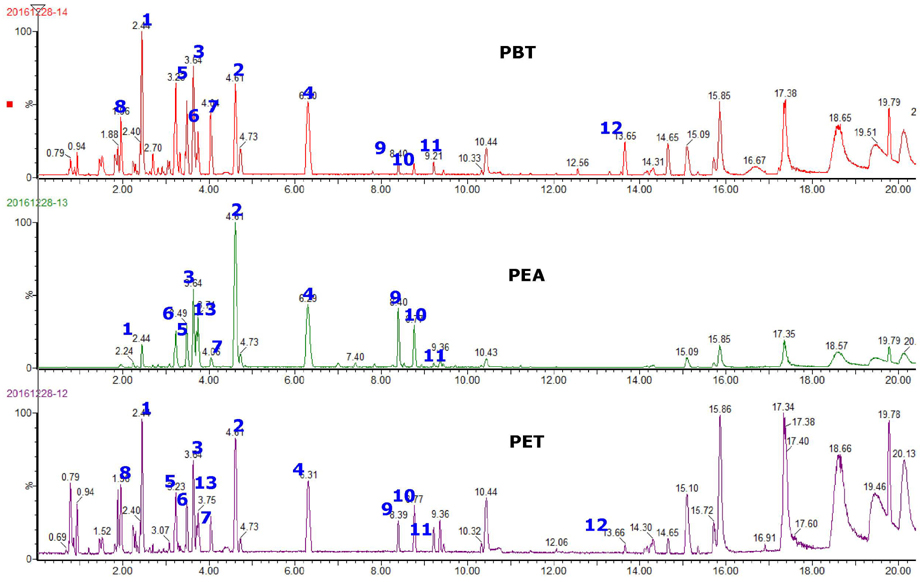
Ultraviolet radiation (UV) is a major cause of skin photoaging. Previous studies reported that ethanol extract (PET) of Prunus persica (L.) Batsch flowers (PPF, peach flowers) and its subfractions, particularly the ethylacetate (PEA) and n-butanol extracts (PBT), have potent antioxidant activity and attenuate the UV-induced matrix metalloproteinase (MMP) expression in human skin cells. In this study, we investigated the protective activity of PPF extract against UV-induced photoaging in a mouse model.
MATERIALS/METHODS
Hairless mice were treated with PET or a mixture of PEA and PBT either topically or orally along with UV irradiation. Histological changes and biochemical alterations of mouse skin were examined. Major phenolic compounds in PPF extract were analyzed using an ACQUITY UPLC system.
RESULTS
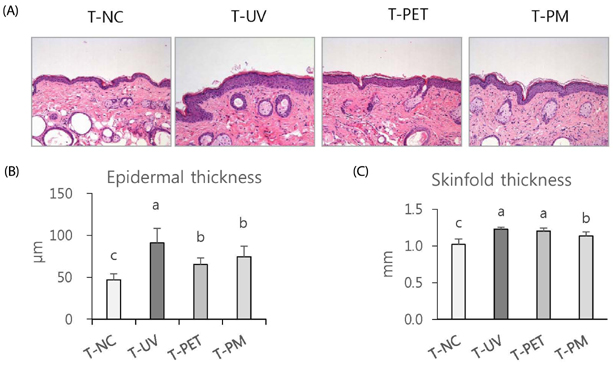
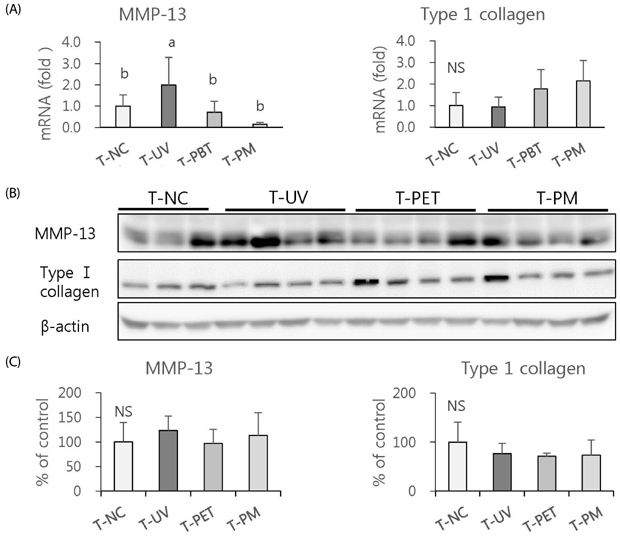
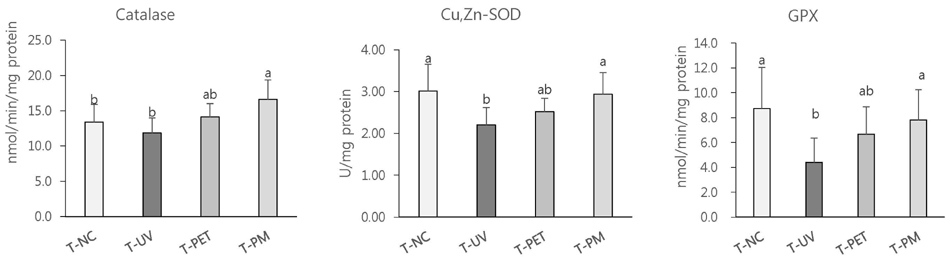
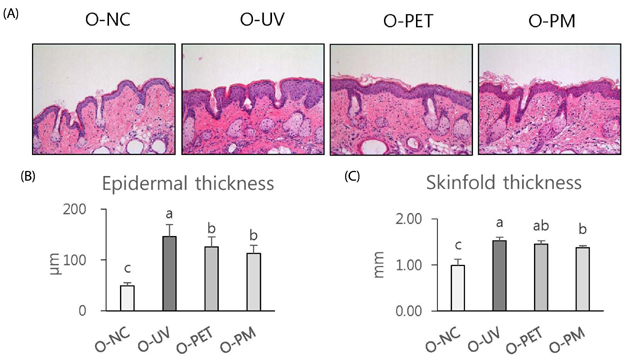
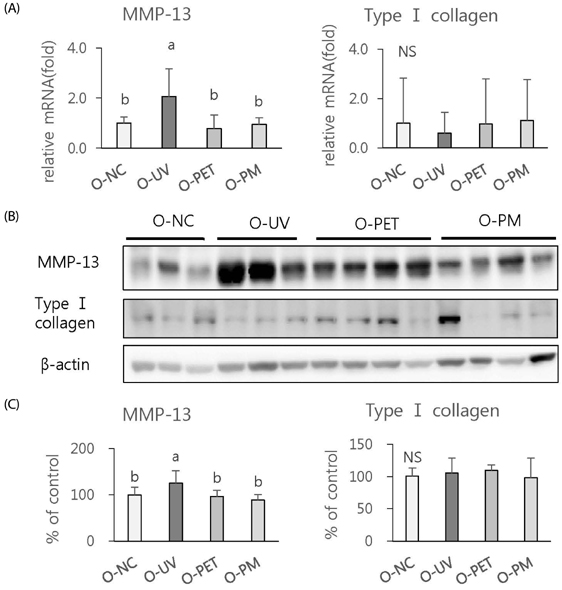
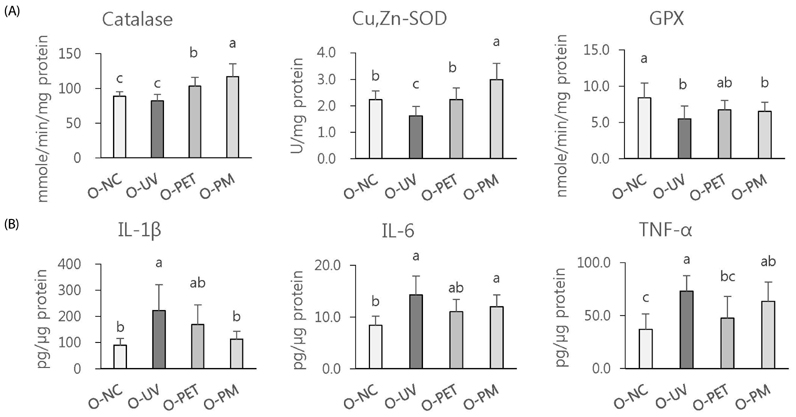
The overall effects of topical and oral treatments with PPF extract on the UV-induced skin responses exhibited similar patterns. In both experiments, the mixture of PEA and PBT significantly inhibited the UV-induced skin and epidermal thickening, while PET inhibited only the UV-induced epidermal thickening. Treatment of PET or the mixture of PEA and PBT significantly inhibited the UV-induced MMP-13 expression, but not typeâ… collagen expression. Topical treatment of the mixture of PEA and PBT with UV irradiation significantly elevated catalase, superoxide dismutase (SOD) and glutathione-peroxidase (GPx) activities in the skin compared to those in the UV irradiated control group, while oral treatment of the mixture of PEA and PBT or PET elevated only catalase and SOD activities, but not GPx. Thirteen phytochemical compounds including 4-O-caffeoylquinic acid, cimicifugic acid E and B, quercetin-3-O-rhamnoside and kaempferol glycoside derivatives were identified in the PPF extract.
CONCLUSIONS
These results demonstrate that treatment with PET or the mixture of PEA and PBT, both topically or orally, attenuates UV-induced photoaging via the cooperative interactions of phenolic components having anti-oxidative and collagen-protective activities.
Keyword
MeSH Terms
Figure
Reference
-
1. Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signaling. Nat Rev Cancer. 2004; 4:23–35.
Article2. Fernández-García E. Skin protection against UV light by dietary antioxidants. Food Funct. 2014; 5:1994–2003.
Article3. Kim M, Park YG, Lee HJ, Lim SJ, Nho CW. Youngiasides A and C isolated from Youngia denticulatum inhibit UVB-induced MMP expression and promote typeⅠ procollagen production via repression of MAPK/AP-1/NF-kB and activation of AMPK/Nrf2 in HaCat cells and human dermal fibroblasts. J Agric Food Chem. 2015; 63:5428–5438.
Article4. Yang HM, Ham YM, Yoon WJ, Roh SW, Jeon YJ, Oda T, Kang SM, Kang MC, Kim EA, Kim D, Kim KN. Quercitrin protects against ultraviolet B-induced cell death in vitro and in an in vivo zebrafish model. J Photochem Photobiol B. 2012; 114:126–131.
Article5. Zhan JY, Wang XF, Liu YH, Zhang ZB, Wang L, Chen JN, Huang S, Zeng HF, Lai XP. Andrographolide sodium bisulfate prevents UV-induced skin photoaging through inhibiting oxidative stress and inflammation. Mediators Inflamm. 2016; 3271451.
Article6. Kim J, Lee CW, Kim EK, Lee SJ, Park NH, Kim HS, Kim HK, Char K, Jang YP, Kim JW. Inhibition effect of Gynura procumbens extract on UVB- induced matrix-metalloproteinase expression in human dermal fibroblasts. J Ethnopharmacol. 2011; 137:427–433.
Article7. Chiang HM, Chen HC, Lin TJ, Shih IC, Wen KC. Michelia alba extract attenuates UVB-induced expression of matrix metalloproteinases via MAP kinase pathway in human dermal fibroblasts. Food Chem Toxicol. 2012; 50:4260–4269.
Article8. Hwang E, Park SY, Lee HJ, Lee TY, Sun ZW, Yi TH. Gallic acid regulates skin photoaging in UVB-exposed fibroblast and hairless mice. Phytother Res. 2014; 28:1778–1788.
Article9. Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009; 14:20–24.
Article10. Hong YF, Lee HY, Jung BJ, Jang S, Chung DK, Kim H. Lipoteichoic acid isolated from Lactobacillus plantarum down-regulates UV-induced MMP-1 expression and up-regulates typeⅠ procollagen through the inhibition of reactive oxygen species generation. Mol Immunol. 2015; 67:248–255.
Article11. Masaki H. Role of antioxidants in the skin: anti-aging effects. J Dermatol Sci. 2010; 58:85–90.
Article12. Park JE, Pyun HB, Woo SW, Jeong JH, Hwang JK. The protective effect of Kaempferia parviflora extract on UVB-induced skin photoaging in hairless mice. Photodermatol Photoimmunol Photomed. 2014; 30:237–245.
Article13. Yoo HG, Lee BH, Kim W, Lee JS, Kim GH, Chun OK, Koo SI, Kim DO. Lithospermum erythrorhizon extract protects keratinocytes and fibroblasts against oxidative stress. J Med Food. 2014; 17:1189–1196.
Article14. Bae JS, Han M, Shin HS, Kim MK, Shin CY, Lee DH, Chung JH. Perilla frutescens leaves extract ameliorates ultraviolet radiation-induced extracellular matrix damage in human dermal fibroblasts and hairless mice skin. J Ethnopharmacol. 2017; 195:334–342.
Article15. Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002; 138:1462–1470.
Article16. Lim JY, Kim OK, Lee J, Lee MJ, Kang N, Hwang JK. Protective effect of the standardized green tea seed extract on UVB-induced skin photoaging in hairless mice. Nutr Res Pract. 2014; 8(4):398–403.
Article17. Katiyar SK, Korman NJ, Mukhtar H, Agarwal R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J Natl Cancer Inst. 1997; 89:556–566.
Article18. Bae JY, Choi JS, Kang SW, Lee YJ, Park J, Kang YH. Dietary compound ellagic acid alleviates skin wrinkle and inflammation induced by UV-B irradiation. Exp Dermatol. 2010; 19:e182–e190.
Article19. Divya SP, Wang X, Pratheeshkumar P, Son YO, Roy RV, Kim D, Dai J, Hitron JA, Wang L, Asha P, Shi X, Zhang Z. Blackberry extract inhibits UVB-induced oxidative damage and inflammation through MAP kinases and NF-κB signaling pathways in SKH-1 mice skin. Toxicol Appl pharmacol. 2015; 284:92–99.
Article20. Patwardhan J, Bhatt P. Ultraviolet-B protective effect of flavonoids from Eugenia caryophylata on human dermal fibroblast cells. Pharmacogn Mag. 2015; 11:S397–S406.21. Lee JY, An BJ. Anti-oxidant and anti-inflammation activities of Prunus persica Flos. J Appl Biol Chem. 2010; 53:162–169.
Article22. Kim YH, Yang HE, Park BK, Heo MY, Jo BK, Kim HP. The extract of the flowers of Prunus persica, a new cosmetic ingredient, protects against solar ultraviolet-induced skin damage in vivo. J Cosmet Sci. 2002; 53:27–34.23. Li C, Wang MH. Antioxidant activity of peach blossom extracts. J Korean Soc Appl Biol Chem. 2011; 54:46–53.
Article24. Lee JY, An BJ. Antioxidant and anti-inflammatory effects of fractions from Pruni persicae Flos. Korean J Herbol. 2012; 27:55–63.
Article25. Kwak CS, Choi HI. In vitro antioxidant and anti-inflammatory activities of ethanol extract and sequential fractions of floweres of Prunus persica in LPS-stimulated RAW 264.7 macrophages. J Korean Soc Food Sci Nutr. 2015; 44:1439–1449.
Article26. Kwak CS, Yang J. Prevention effect of prunus persica flos extract from reactive oxygen species generation and matrix metalloproteinases production induced by UVB irradiation in human skin cells. Asian J Beauty Cosmet. 2016; 14:179–190.
Article27. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408.
Article28. Aebi H. Catalase in vitro. Methods Enzymol. 1984; 105:121–126.29. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974; 47:469–474.
Article30. Tappel AL. Glutathione peroxidase and hydroperoxides. Methods Enzymol. 1978; 52:506–513.31. Wang XF, Huang YF, Wang L, Xu LQ, Yu XT, Liu YH, Li CL, Zhan JY, Su ZR, Chen JN, Zeng HF. Photo-protective activity of pogostone against UV-induced skin premature aging in mice. Exp Gerontol. 2016; 77:76–86.
Article32. Liu JC, Jiao ZG, Yang WB, Zhang CL, Liu H, Lv ZZ. Variation in phenolics, flavonoids, antioxidant and tyrosinase inhibitory activity of peach blossoms at different developmental stages. Molecules. 2015; 20:20460–20472.
Article33. Lee KO, Kim SN, Kim YC. Anti-wrinkle effects of water extracts of teas in hairless mouse. Toxicol Res. 2014; 30:283–289.
Article34. Song JH, Bae EY, Choi G, Hyun JW, Lee MY, Lee HW, Chae S. Protective effect of mango (Mangifera indica L.) against UVB-induced skin aging in hairless mice. Photodermatol Photoimmunol Photomed. 2013; 29:84–89.
Article35. Rhodes LE, Belgi G, Parslew R, McLoughlin L, Clough GF, Friedmann PS. Ultraviolet-B-induced erythema is mediated by nitric oxide and prostaglandin E2 in combination. J Invest Dermatol. 2001; 117:880–885.
Article36. Pillai S, Oresajo C, Hayward J. Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation - a review. Int J Cosmet Sci. 2005; 27:17–34.
Article37. Vayalil PK, Mittal A, Hara Y, Elmets CA, Katiyar SK. Green tea polyphenols prevent ultraviolet light-induced oxidative damage and matrix metalloproteinases expression in mouse skin. J Invest Dermatol. 2004; 122:1480–1487.
Article38. Hassani D, Liu HL, Chen YN, Wan ZB, Zhuge Q, Li SX. Analysis of biochemical compounds and differentially expressed genes of the anthocyanin biosynthetic pathway in variegated peach flowers. Genet Mol Res. 2015; 14:13425–13436.
Article39. Jin UH, Lee JY, Kang SK, Kim JK, Park WH, Kim JG, Moon SK, Kim CH. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: isolation and identification from methanol extract of Euonymus alatus. Life Sci. 2005; 77:2760–2769.
Article40. Kusano A, Seyama Y, Nagai M, Shibano M, Kusano G. Effects of fukinolic acid and cimicifugic acids from Cimicifuga species on collagenolytic activity. Biol Pharm Bull. 2001; 24:1198–1201.
Article41. Yin Y, Li W, Son YO, Sun L, Lu J, Kim D, Wang X, Yao H, Wang L, Pratheeshkumar P, Hitron AJ, Luo J, Gao N, Shi X, Zhang Z. Quercitrin protects skin from UVB-induced oxidative damage. Toxicol Appl Pharmacol. 2013; 269:89–99.
Article42. Vicentini FT, He T, Shao Y, Fonseca MJ, Verri WA Jr, Fisher GJ, Xu Y. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-kB pathway. J Dermatol Sci. 2011; 61:162–168.
Article43. Maini S, Fahlman BM, Krol ES. Flavonols protect against UV radiation-induced thymine dimer formation in an artificial skin mimic. J Pharm Pharm Sci. 2015; 18:600–615.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective effects of red orange (Citrus sinensis [L.] Osbeck [Rutaceae]) extract against UVA-B radiation-induced photoaging in Skh:HR-2 mice
- Prevention of UV-Induced Skin Damages by 11,14,17-Eicosatrienoic Acid in Hairless Mice In Vivo
- Dietary effect of green tea extract on epidermal levels of skin pH related factors, lactate dehydrogenase protein expression and activity in UV-irradiated hairless mice
- Dietary effect of green tea extract on hydration improvement and metabolism of free amino acid generation in epidermis of UV-irradiated hairless mice
- Topical Application of Selenium Can Significantly Relieve UV-induced Skin Aging in Hairless Mice