Nutr Res Pract.
2018 Feb;12(1):13-19. 10.4162/nrp.2018.12.1.13.
Anti-neuroinflammatory effects of ethanolic extract of black chokeberry (Aronia melanocapa L.) in lipopolysaccharide-stimulated BV2 cells and ICR mice
- Affiliations
-
- 1Department of Physiology, School of Medicine, Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul 05029, Korea. gostop48@konkuk.ac.kr
- 2Department of Biotechnology, College of Engineering, Daegu University, Gyeongsan 38453, Korea.
- 3Department of Marine Life Science, Jeju National University, Jeju 63243, Korea.
- 4Department of Anatomy, College of Korean Medicine, Semyung University, Jecheon 27136, Korea.
- 5Department of Anatomy, College of Korean Medicine, Dongguk University, Gyeongju 38066, Korea.
- 6Department of Bio-Science, College of Natural Science, Dongguk University, Dongdae-ro 123, Gyeongju, Gyeongbuk 38066, Korea. dwleebio@dongguk.ac.kr
- KMID: 2429014
- DOI: http://doi.org/10.4162/nrp.2018.12.1.13
Abstract
- BACKGROUND/OBJECTIVES
One of the mechanisms considered to be prevalent in the development of Alzheimer's disease (AD) is hyper-stimulation of microglia. Black chokeberry (Aronia melanocapa L.) is widely used to treat diabetes and atherosclerosis, and is known to exert anti-oxidant and anti-inflammatory effects; however, its neuroprotective effects have not been elucidated thus far.
MATERIALS/METHODS
We undertook to assess the anti-inflammatory effect of the ethanolic extract of black chokeberry friut (BCE) in BV2 cells, and evaluate its neuroprotective effect in the lipopolysaccharide (LPS)-induced mouse model of AD.
RESULTS
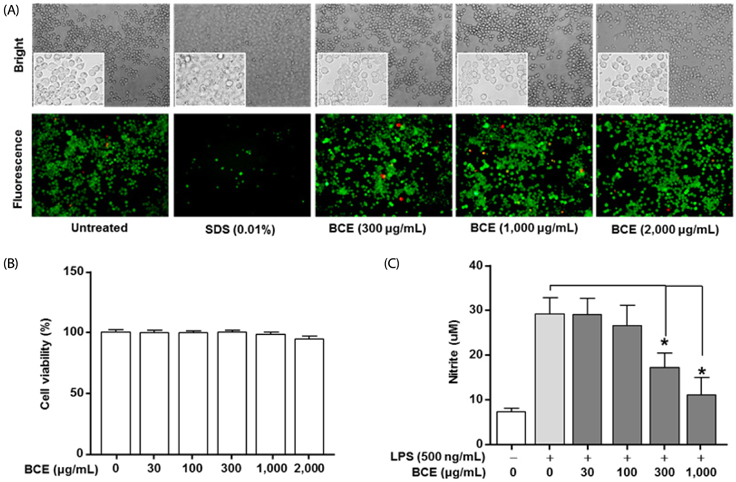
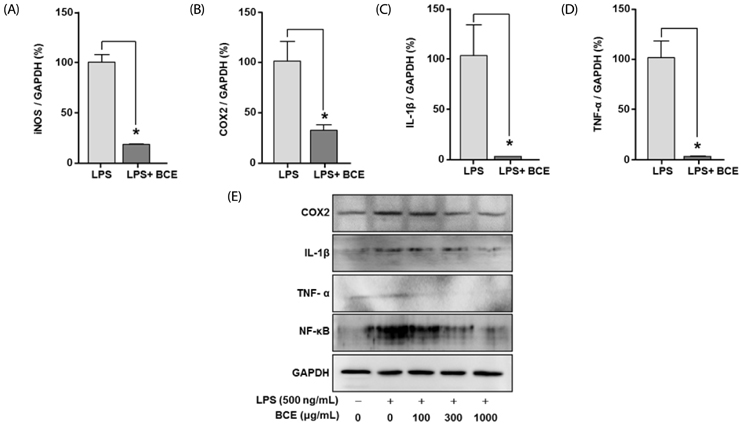
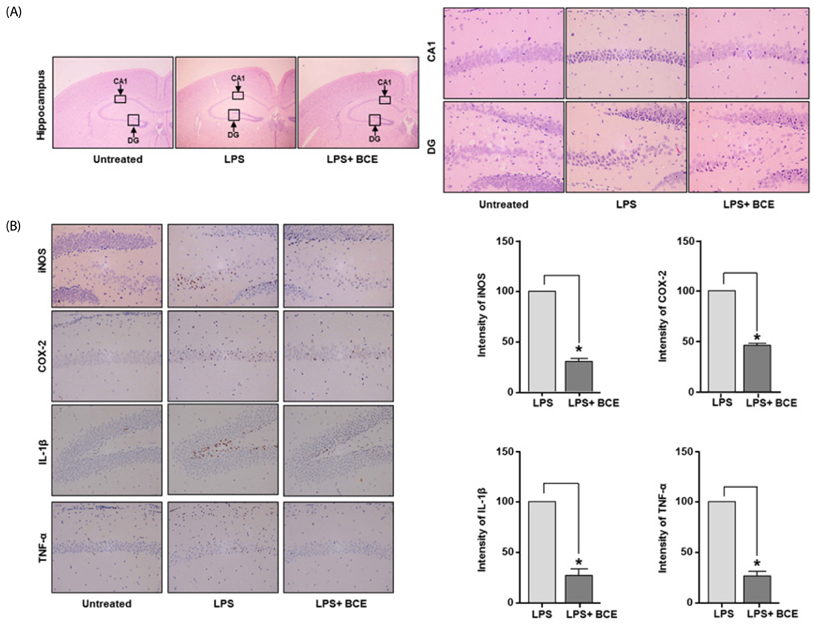
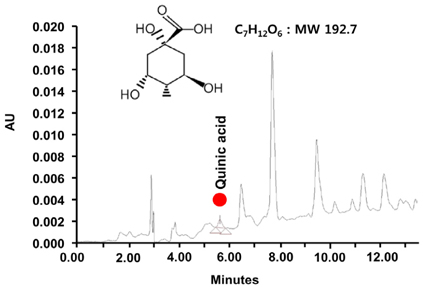
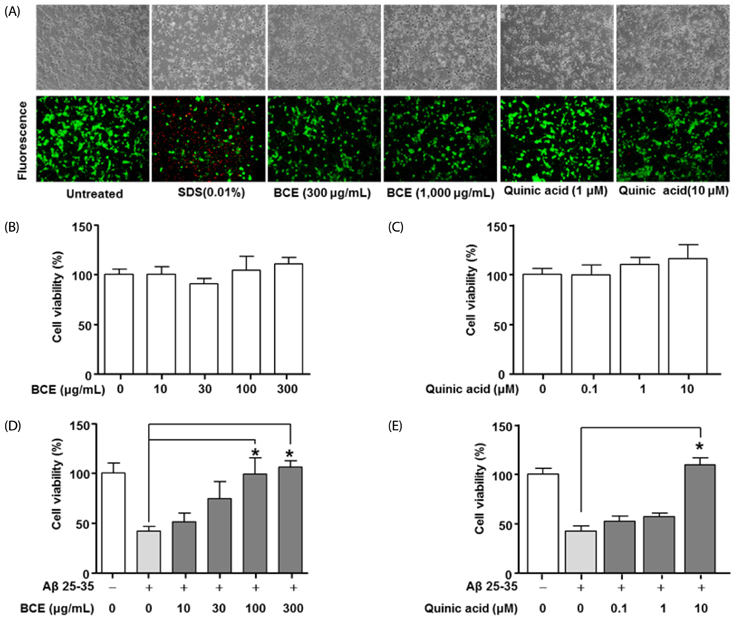
Following stimulation of BV2 cells by LPS, exposure to BCE significantly reduced the generation of nitric oxide as well as mRNA levels of numerous inflammatory factors such as inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2), interleukin 1 beta (IL-1β), and tumor necrosis factor alpha (TNF-α). In addition, AD was induced in a mouse model by intraperitoneal injection of LPS (250 µg/kg), subsequent to which we investigated the neuroprotective effects of BCE (50 mg/kg) on brain damage. We observed that BCE significantly reduced tissue damage in the hippocampus by downregulating iNOS, COX-2, and TNF-α levels. We further identified the quinic acids in BCE using liquid chromatography-mass spectrometry (LCMS). Furthermore, we confirmed the neuroprotective effect of BCE and quinic acid on amyloid beta-induced cell death in rat hippocampal primary neurons.
CONCLUSIONS
Our findings suggest that black chokeberry has protective effects against the development of AD.
Keyword
MeSH Terms
-
Alzheimer Disease
Amyloid
Animals
Atherosclerosis
Brain
Cell Death
Cyclooxygenase 2
Ethanol*
Hippocampus
Inflammation
Injections, Intraperitoneal
Interleukin-1beta
Mice
Mice, Inbred ICR*
Microglia
Neurons
Neuroprotective Agents
Nitric Oxide
Nitric Oxide Synthase Type II
Phytochemicals
Quinic Acid
Rats
RNA, Messenger
Spectrum Analysis
Tumor Necrosis Factor-alpha
Amyloid
Cyclooxygenase 2
Ethanol
Interleukin-1beta
Neuroprotective Agents
Nitric Oxide
Nitric Oxide Synthase Type II
Quinic Acid
RNA, Messenger
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Obisesan TO, Gillum RF, Johnson S, Umar N, Williams D, Bond V, Kwagyan J. Neuroprotection and neurodegeneration in Alzheimer's disease: role of cardiovascular disease risk factors, implications for dementia rates, and prevention with aerobic exercise in African Americans. Int J Alzheimers Dis. 2012; 2012:568382.
Article2. Ramírez BG, Blázquez C, Gómez del, Guzmán M, de Ceballos ML. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005; 25:1904–1913.
Article3. Katzman R. Alzheimer's disease. N Engl J Med. 1986; 314:964–973.
Article4. Choonara YE, Pillay V, du Toit LC, Modi G, Naidoo D, Ndesendo VM, Sibambo SR. Trends in the molecular pathogenesis and clinical therapeutics of common neurodegenerative disorders. Int J Mol Sci. 2009; 10:2510–2557.
Article5. Murphy MP, LeVine H 3rd. Alzheimer's disease and the amyloid-beta peptide. J Alzheimers Dis. 2010; 19:311–323.6. LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci. 2007; 8:499–509.7. Zotova E, Nicoll JA, Kalaria R, Holmes C, Boche D. Inflammation in Alzheimer's disease: relevance to pathogenesis and therapy. Alzheimers Res Ther. 2010; 2:1.
Article8. Chen Z, Jalabi W, Shpargel KB, Farabaugh KT, Dutta R, Yin X, Kidd GJ, Bergmann CC, Stohlman SA, Trapp BD. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J Neurosci. 2012; 32:11706–11715.
Article9. Choi SK, Park YS, Choi DK, Chang HI. Effects of astaxanthin on the production of NO and the expression of COX-2 and iNOS in LPS-stimulated BV2 microglial cells. J Microbiol Biotechnol. 2008; 18:1990–1996.10. Bamberger ME, Landreth GE. Microglial interaction with beta-amyloid: implications for the pathogenesis of Alzheimer's disease. Microsc Res Tech. 2001; 54:59–70.
Article11. Lee CY, Landreth GE. The role of microglia in amyloid clearance from the AD brain. J Neural Transm (Vienna). 2010; 117:949–960.
Article12. Nimmervoll B, White R, Yang JW, An S, Henn C, Sun JJ, Luhmann HJ. LPS-induced microglial secretion of TNFα increases activity-dependent neuronal apoptosis in the neonatal cerebral cortex. Cereb Cortex. 2013; 23:1742–1755.
Article13. Wang WY, Tan MS, Yu JT, Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer's disease. Ann Transl Med. 2015; 3:136.14. Dai Q, Borenstein AR, Wu Y, Jackson JC, Larson EB. Fruit and vegetable juices and Alzheimer's disease: the Kame Project. Am J Med. 2006; 119:751–759.
Article15. Mao J, Huang S, Liu S, Feng XL, Yu M, Liu J, Sun YE, Chen G, Yu Y, Zhao J, Pei G. A herbal medicine for Alzheimer's disease and its active constituents promote neural progenitor proliferation. Aging Cell. 2015; 14:784–796.
Article16. Dragan S, Andrica F, Serban MC, Timar R. Polyphenols-rich natural products for treatment of diabetes. Curr Med Chem. 2015; 22:14–22.
Article17. Zapolska-Downar D, Bryk D, Małecki M, Hajdukiewicz K, Sitkiewicz D. Aronia melanocarpa fruit extract exhibits anti-inflammatory activity in human aortic endothelial cells. Eur J Nutr. 2012; 51:563–572.
Article18. Jurikova T, Mlcek J, Skrovankova S, Sumczynski D, Sochor J, Hlavacova I, Snopek L, Orsavova J. Fruits of black chokeberry aronia melanocarpa in the prevention of chronic diseases. Molecules. 2017; 22:E944.
Article19. Lee KP, Kim JE, Park WH. Cytoprotective effect of rhamnetin on miconazole-induced H9c2 cell damage. Nutr Res Pract. 2015; 9:586–591.
Article20. Paulrayer A, Adithan A, Lee JH, Moon KH, Kim DG, Im SY, Kang CW, Kim NS, Kim JH. Aronia melanocarpa (black chokeberry) reduces ethanol-induced gastric damage via regulation of HSP-70, NF-κB, and MCP-1 signaling. Int J Mol Sci. 2017; 18:E1195.
Article21. Lee KP, Choi NH, Sudjarwo GW, Ahn SH, Park IS, Lee SR, Hong H. Protective effect of areca catechu leaf ethanol extract against ethanol-induced gastric ulcers in ICR mice. J Med Food. 2016; 19:127–132.
Article22. Gasparini L, Rusconi L, Xu H, del Soldato P, Ongini E. Modulation of beta-amyloid metabolism by non-steroidal anti-inflammatory drugs in neuronal cell cultures. J Neurochem. 2004; 88:337–348.
Article23. Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, Hong JT. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008; 5:37.
Article24. Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, Citron M, Landreth G. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer's disease. J Neurosci. 2003; 23:7504–7509.
Article25. Granado-Lorencio F, Hernández-Alvarez E. Functional foods and health effects: a nutritional biochemistry perspective. Curr Med Chem. 2016; 23:2929–2957.
Article26. Aronson JK. Defining ‘nutraceuticals’: neither nutritious nor pharmaceutical. Br J Clin Pharmacol. 2017; 83:8–19.
Article27. Asiimwe N, Yeo SG, Kim MS, Jung J, Jeong NY. Nitric oxide: exploring the contextual link with Alzheimer's disease. Oxid Med Cell Longev. 2016; 2016:7205747.
Article28. Danysz W, Parsons CG. Alzheimer's disease, β-amyloid, glutamate, NMDA receptors and memantine--searching for the connections. Br J Pharmacol. 2012; 167:324–352.
Article29. Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002; 298:789–791.
Article30. Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016; 8:595–608.
Article31. Pero RW, Lund H, Leanderson T. Antioxidant metabolism induced by quinic acid. Increased urinary excretion of tryptophan and nicotinamide. Phytother Res. 2009; 23:335–346.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- First Report of Leaf Spot Caused by Alternaria tenuissima on Black Chokeberry (Aronia melanocarpa) in Korea
- 2-Aryl Propionic Acid Amide Modification of Naproxen and Ibuprofen Dimers for Anti-neuroinflammatory Activity in BV2 mouse Microglial Cells
- Viridicatol from Marine-derived Fungal Strain Penicillium sp. SF-5295 Exerts Anti-inflammatory Effects through Inhibiting NF-kappaB Signaling Pathway on Lipopolysaccharide-induced RAW264.7 and BV2 Cells
- Antineuroinflammatory Effects of 7,3’,4’-Trihydroxyisoflavone in Lipopolysaccharide-Stimulated BV2 Microglial Cells through MAPK and NF-κB Signaling Suppression
- Synthetic 3′,4′-Dihydroxyflavone Exerts Anti-Neuroinflammatory Effects in BV2 Microglia and a Mouse Model