Yonsei Med J.
2018 Sep;59(7):816-826. 10.3349/ymj.2018.59.7.816.
XIST Induced by JPX Suppresses Hepatocellular Carcinoma by Sponging miR-155-5p
- Affiliations
-
- 1Department of Gastroenterology, First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China. uynixoahzday@sina.com
- KMID: 2428907
- DOI: http://doi.org/10.3349/ymj.2018.59.7.816
Abstract
- PURPOSE
The influence of X-inactive specific transcript (XIST) and X-chromosome inactivation associated long non-coding RNAs (lncRNAs) just proximal to XIST (JPX) on hepatocellular carcinoma (HCC) remains controversial in light of previous reports, which the present study aimed to verify.
MATERIALS AND METHODS
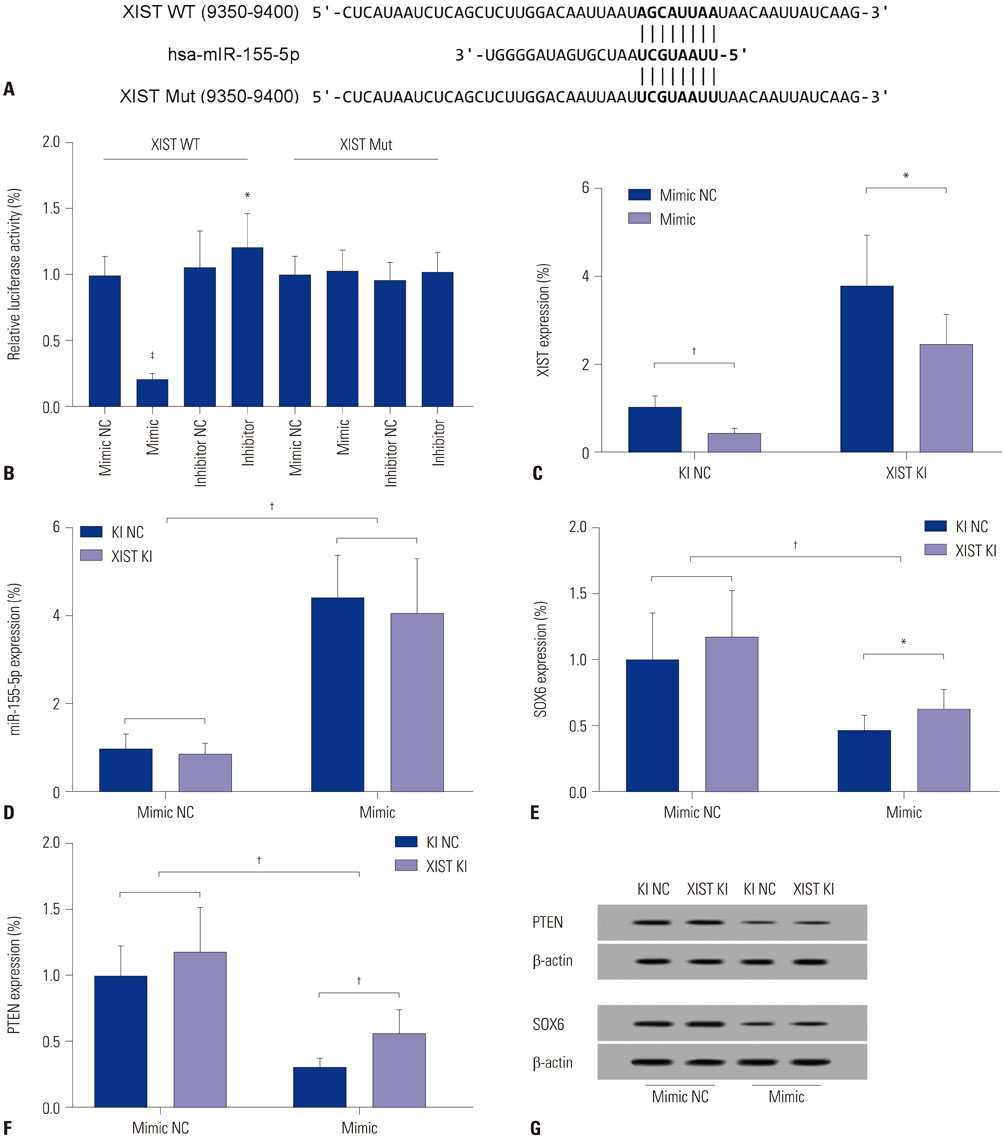
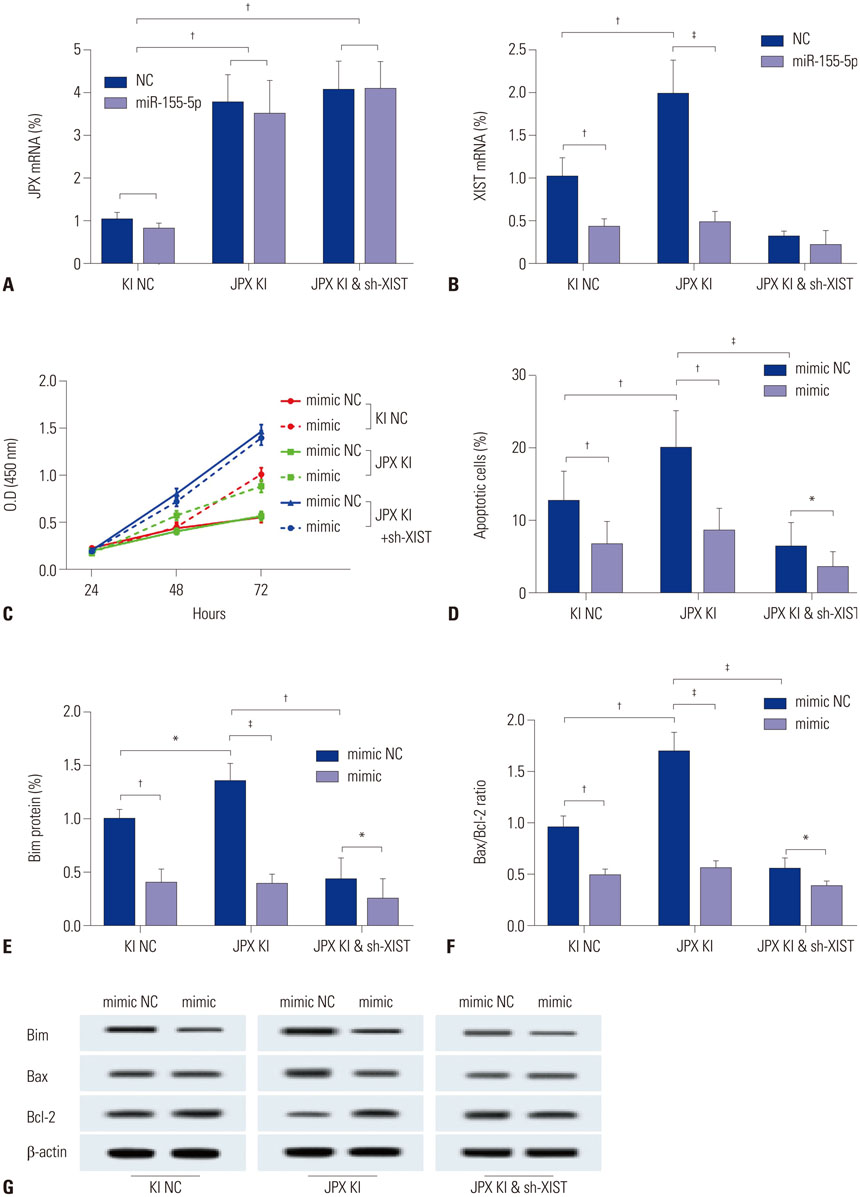
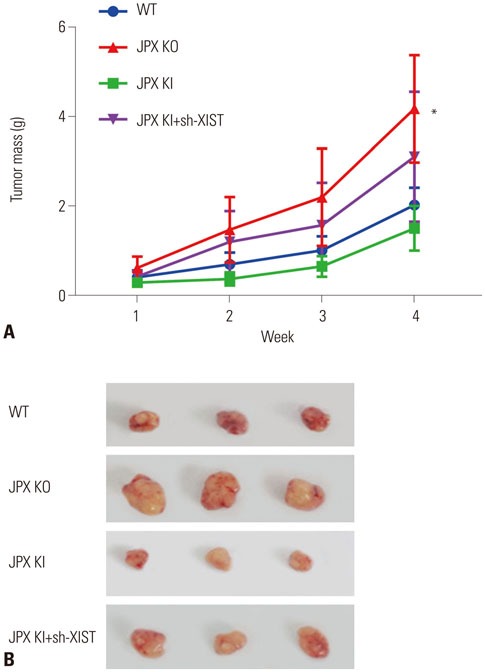
The DIANA lncRNA-microRNA (miRNA) interaction database was used to explore miRNA interactions with JPX or XIST. JPX, XIST, and miR-155-5p expression levels in paired HCC specimens and adjacent normal tissue were analyzed by RT-qPCR. Interaction between XIST and miR-155-5p was verified by dual luciferase reporter assay. Expression levels of miR-155-5p and its known target genes, SOX6 and PTEN, were verified by RT-qPCR and Western blot in HepG2 cells with or without XIST knock-in. The potential suppressive role of XIST and JPX on HCC was verified by cell functional assays and tumor formation assay using a xenograft model.
RESULTS
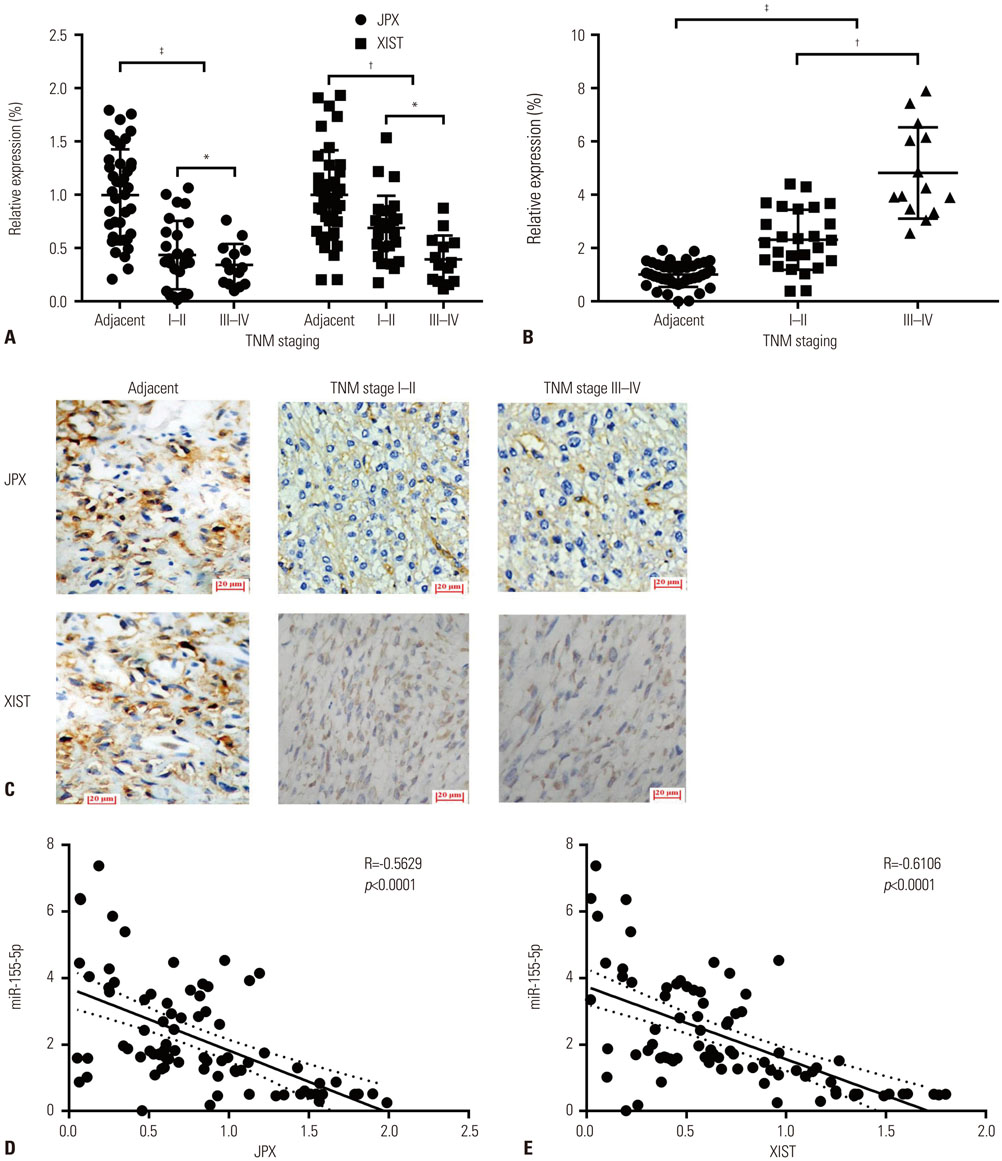
JPX and XIST expression was significantly decreased in HCC pathologic specimens, compared to adjacent tissue, which correlated with HCC progression and increased miR-155-5p expression. Dual luciferase reporter assay revealed XIST as a direct target of miR-155-5p. XIST knock-in significantly reduced miR-155-5p expression level and increased that of SOX6 and PTEN, while significantly inhibiting HepG2 cell growth in vitro, which was partially reversed by miR-155-5p mimic transfection. JPX knock-in significantly increased XIST expression and inhibited HepG2 cell growth in vitro or tumor formation in vivo in a XIST dependent manner.
CONCLUSION
JPX and XIST play a suppressive role in HCC. JPX increases expression levels of XIST in HCC cells, which suppresses HCC development by sponging the cancer promoting miR-155-5p.
Keyword
MeSH Terms
Figure
Reference
-
1. Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014; 505:344–352.
Article2. Karreth FA, Pandolfi PP. ceRNA cross-talk in cancer: when ce-bling rivalries go awry. Cancer Discov. 2013; 3:1113–1121.
Article3. Yuan SX, Wang J, Yang F, Tao QF, Zhang J, Wang LL, et al. Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma by derepression of CTNNB1. Hepatology. 2016; 63:499–511.
Article4. Fu WM, Zhu X, Wang WM, Lu YF, Hu BG, Wang H, et al. Hotair mediates hepatocarcinogenesis through suppressing miRNA-218 expression and activating P14 and P16 signaling. J Hepatol. 2015; 63:886–895.
Article5. Cui M, Xiao Z, Wang Y, Zheng M, Song T, Cai X, et al. Long non-coding RNA HULC modulates abnormal lipid metabolism in hepatoma cells through an miR-9-mediated RXRA signaling pathway. Cancer Res. 2015; 75:846–857.
Article6. Ma X, Yuan T, Yang C, Wang Z, Zang Y, Wu L, et al. X-inactive-specific transcript of peripheral blood cells is regulated by exosomal Jpx and acts as a biomarker for female patients with hepatocellular carcinoma. Ther Adv Med Oncol. 2017; 9:665–677.
Article7. Ma W, Wang H, Jing W, Zhou F, Chang L, Hong Z, et al. Downregulation of long non-coding RNAs JPX and XIST is associated with the prognosis of hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2017; 41:163–170.
Article8. Mo Y, Lu Y, Wang P, Huang S, He L, Li D, et al. Long non-coding RNA XIST promotes cell growth by regulating miR-139-5p/PDK1/AKT axis in hepatocellular carcinoma. Tumour Biol. 2017; 39:1010428317690999.
Article9. Li C, Wan L, Liu Z, Xu G, Wang S, Su Z, et al. Long non-coding RNA XIST promotes TGF-β-induced epithelial-mesenchymal transition by regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer. Cancer Lett. 2018; 418:185–195.
Article10. Zhang Q, Chen B, Liu P, Yang J. XIST promotes gastric cancer (GC) progression through TGF-β1 via targeting miR-185. J Cell Biochem. 2018; 119:2787–2796.
Article11. Du Y, Weng XD, Wang L, Liu XH, Zhu HC, Guo J, et al. LncRNA XIST acts as a tumor suppressor in prostate cancer through sponging miR-23a to modulate RKIP expression. Oncotarget. 2017; 8:94358–94370.
Article12. Xiao C, Sharp JA, Kawahara M, Davalos AR, Difilippantonio MJ, Hu Y, et al. The XIST noncoding RNA functions independently of BRCA1 in X inactivation. Cell. 2007; 128:977–989.
Article13. Liu X, Cui L, Hua D. Long non-coding RNA XIST regulates miR-137-EZH2 axis to promote tumor metastasis in colorectal cancer. Oncol Res. 2018; 03. 01. [Epub]. DOI: 10.3727/096504018X15195193936573.
Article14. Huang YS, Chang CC, Lee SS, Jou YS, Shih HM. Xist reduction in breast cancer upregulates AKT phosphorylation via HDAC3-mediated repression of PHLPP1 expression. Oncotarget. 2016; 7:43256–43266.
Article15. Yan XL, Jia YL, Chen L, Zeng Q, Zhou JN, Fu CJ, et al. Hepatocellular carcinoma-associated mesenchymal stem cells promote hepatocarcinoma progression: role of the S100A4-miR155-SOCS1-MMP9 axis. Hepatology. 2013; 57:2274–2286.
Article16. Wang B, Majumder S, Nuovo G, Kutay H, Volinia S, Patel T, et al. Role of microRNA-155 at early stages of hepatocarcinogenesis induced by choline-deficient and amino acid-defined diet in C57BL/6 mice. Hepatology. 2009; 50:1152–1161.
Article17. Chen G, Wang D, Zhao X, Cao J, Zhao Y, Wang F, et al. miR-155-5p modulates malignant behaviors of hepatocellular carcinoma by directly targeting CTHRC1 and indirectly regulating GSK-3β-involved Wnt/β-catenin signaling. Cancer Cell Int. 2017; 17:118.
Article18. Fu X, Wen H, Jing L, Yang Y, Wang W, Liang X, et al. MicroRNA-155-5p promotes hepatocellular carcinoma progression by suppressing PTEN through the PI3K/Akt pathway. Cancer Sci. 2017; 108:620–631.
Article19. Wong CM, Tsang FH, Ng IO. Non-coding RNAs in hepatocellular carcinoma: molecular functions and pathological implications. Nat Rev Gastroenterol Hepatol. 2018; 15:137–151.
Article20. Pintacuda G, Young AN, Cerase A. Function by structure: spotlights on Xist long non-coding RNA. Front Mol Biosci. 2017; 4:90.
Article21. Robert Finestra T, Gribnau J. X chromosome inactivation: silencing, topology and reactivation. Curr Opin Cell Biol. 2017; 46:54–61.
Article22. Xing Y, Wen X, Ding X, Fan J, Chai P, Jia R, et al. CANT1 lncRNA triggers efficient therapeutic efficacy by correcting aberrant lncing cascade in malignant uveal melanoma. Mol Ther. 2017; 25:1209–1221.
Article23. Yildirim E, Kirby JE, Brown DE, Mercier FE, Sadreyev RI, Scadden DT, et al. Xist RNA is a potent suppressor of hematologic cancer in mice. Cell. 2013; 152:727–742.
Article24. Jiang H, Zhang H, Hu X, Li W. Knockdown of long non-coding RNA XIST inhibits cell viability and invasion by regulating miR-137/PXN axis in non-small cell lung cancer. Int J Biol Macromol. 2018; 111:623–631.
Article25. Zhang R, Xia T. Long non-coding RNA XIST regulates PDCD4 expression by interacting with miR-21-5p and inhibits osteosarcoma cell growth and metastasis. Int J Oncol. 2017; 51:1460–1470.
Article26. Lin C, Yang L. Long noncoding RNA in cancer: wiring signaling circuitry. Trends Cell Biol. 2018; 28:287–301.
Article27. Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017; 77:3965–3981.
Article28. Chang S, Chen B, Wang X, Wu K, Sun Y. Long non-coding RNA XIST regulates PTEN expression by sponging miR-181a and promotes hepatocellular carcinoma progression. BMC Cancer. 2017; 17:248.
Article29. Sun S, Del Rosario BC, Szanto A, Ogawa Y, Jeon Y, Lee JT. Jpx RNA activates Xist by evicting CTCF. Cell. 2013; 153:1537–1551.
Article30. Tian D, Sun S, Lee JT. The long noncoding RNA, Jpx, is a molecular switch for X chromosome inactivation. Cell. 2010; 143:390–403.
Article31. Romito A, Rougeulle C. Origin and evolution of the long non-coding genes in the X-inactivation center. Biochimie. 2011; 93:1935–1942.
Article32. Chow JC, Hall LL, Clemson CM, Lawrence JB, Brown CJ. Characterization of expression at the human XIST locus in somatic, embryonal carcinoma, and transgenic cell lines. Genomics. 2003; 82:309–322.
Article33. Paraskevopoulou MD, Vlachos IS, Karagkouni D, Georgakilas G, Kanellos I, Vergoulis T, et al. DIANA-LncBase v2: indexing microRNA targets on non-coding transcripts. Nucleic Acids Res. 2016; 44:D231–D238.
Article34. Wang M, Yang F, Qiu R, Zhu M, Zhang H, Xu W, et al. The role of mmu-miR-155-5p-NF-κB signaling in the education of bone marrow-derived mesenchymal stem cells by gastric cancer cells. Cancer Med. 2018; 7:856–868.
Article35. Zhang Y, Wei C, Guo CC, Bi RX, Xie J, Guan DH, et al. Prognostic value of microRNAs in hepatocellular carcinoma: a meta-analysis. Oncotarget. 2017; 8:107237–107257.
Article36. Zhang L, Wang W, Li X, He S, Yao J, Wang X, et al. MicroRNA-155 promotes tumor growth of human hepatocellular carcinoma by targeting ARID2. Int J Oncol. 2016; 48:2425–2434.
Article37. Xie Q, Chen X, Lu F, Zhang T, Hao M, Wang Y, et al. Aberrant expression of microRNA 155 may accelerate cell proliferation by targeting sex-determining region Y box 6 in hepatocellular carcinoma. Cancer. 2012; 118:2431–2442.
Article38. Liu F, Yuan JH, Huang JF, Yang F, Wang TT, Ma JZ, et al. Long non-coding RNA FTX inhibits hepatocellular carcinoma proliferation and metastasis by binding MCM2 and miR-374a. Oncogene. 2016; 35:5422–5434.
Article39. Zhuang LK, Yang YT, Ma X, Han B, Wang ZS, Zhao QY, et al. MicroRNA-92b promotes hepatocellular carcinoma progression by targeting Smad7 and is mediated by long non-coding RNA XIST. Cell Death Dis. 2016; 7:e2203.
Article40. Kristiansen M, Knudsen GP, Maguire P, Margolin S, Pedersen J, Lindblom A, et al. High incidence of skewed X chromosome inactivation in young patients with familial non-BRCA1/BRCA2 breast cancer. J Med Genet. 2005; 42:877–880.
Article41. Manoukian S, Verderio P, Tabano S, Colapietro P, Pizzamiglio S, Grati FR, et al. X chromosome inactivation pattern in BRCA gene mutation carriers. Eur J Cancer. 2013; 49:1136–1141.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long Non-Coding RNA NORAD Inhibits Breast Cancer Cell Proliferation and Metastasis by Regulating miR-155-5p/ SOCS1 Axis
- Long Noncoding RNA Cytoskeleton Regulator RNA Suppresses Apoptosis in Hepatoma Cells by Modulating the miR-125a-5p/ HS1-Associated Protein X-1 Axis to Induce Caspase-9 Inactivation
- Upregulation of long non-coding RNA XIST has anticancer effects on epithelial ovarian cancer cells through inverse downregulation of hsa-miR-214-3p
- Long Intergenic Non-Protein Coding RNA 665 Regulates Viability, Apoptosis, and Autophagy via the MiR-186-5p/MAP4K3 Axis in Hepatocellular Carcinoma
- Overexpression of miR-155-5p Inhibits the Proliferation and Migration of IL-13-Induced Human Bronchial Smooth Muscle Cells by Suppressing TGF-β-Activated Kinase 1/MAP3K7-Binding Protein 2