Clin Endosc.
2018 Sep;51(5):407-415. 10.5946/ce.2018.140.
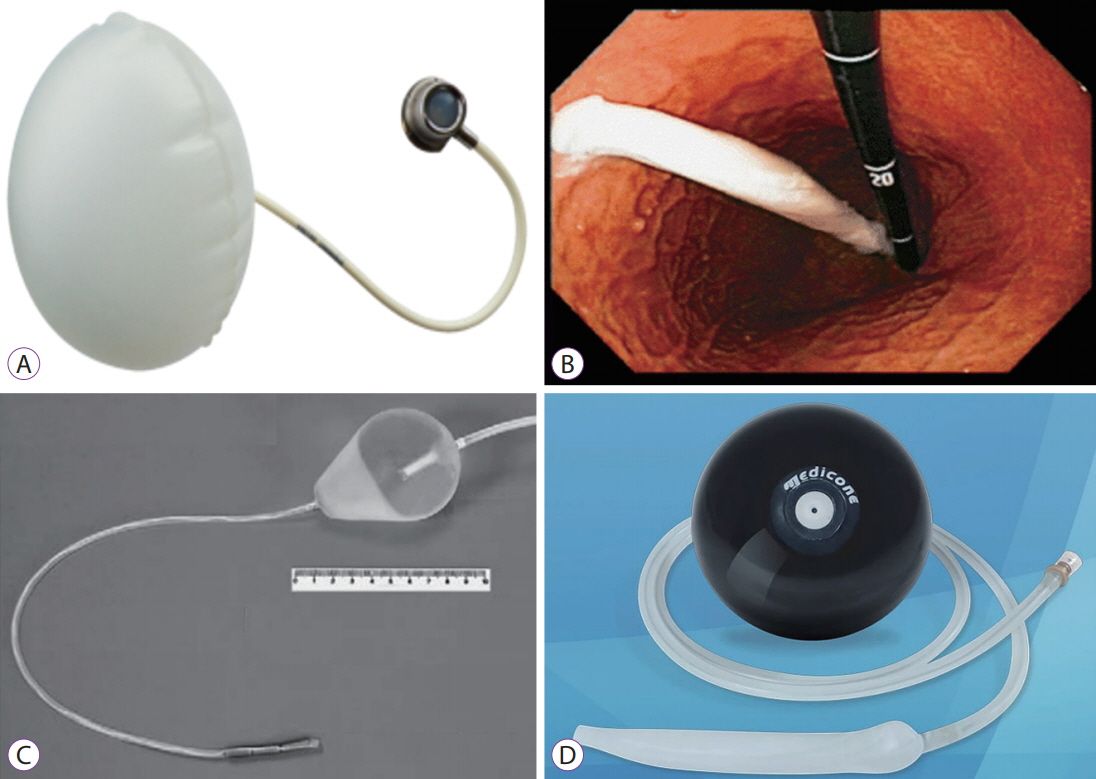
Various Intragastric Balloons Under Clinical Investigation
- Affiliations
-
- 1Division of Gastroenterology and Hepatology, Department of Internal Medicine, Institute of Gastrointestinal Medical Instrument Research, Korea University College of Medicine, Seoul, Korea. mdkorea@gmail.com
- KMID: 2427712
- DOI: http://doi.org/10.5946/ce.2018.140
Abstract
- Obesity is a chronic disease with an exponentially increasing incidence rate, and its negative effects are well documented in numerous studies. As a result, the importance of bariatric therapy cannot be overemphasized, and many bariatric treatment methods with varying mechanisms have been developed. Of the available treatment methods, intragastric balloons, introduced in the 1980s, have been shown to be a safe and effective treatment modality; various intragastric balloon products have been developed and are currently being widely used in clinical settings. However, the disadvantages of intragastric balloons, such as unclear long-term weight loss benefits and complications experienced during insertion and removal, preclude their wider use. In this review, we discuss different intragastric balloon products, focusing on those under clinical investigation, and suggest future research directions.
Keyword
Figure
Cited by 2 articles
-
Effects of intragastric balloon on obesity in obese Korean women for 6 months post removal
Hyeon-Ju Pak, Ha-Neul Choi, Hong-Chan Lee, Jung-Eun Yim
Nutr Res Pract. 2021;15(4):456-467. doi: 10.4162/nrp.2021.15.4.456.The Clinical and Metabolic Effects of Intragastric Balloon on Morbid Obesity and Its Related Comorbidities
Joon Hyun Cho, Mohammad Bilal, Min Cheol Kim, Jonah Cohen
Clin Endosc. 2021;54(1):9-16. doi: 10.5946/ce.2020.302.
Reference
-
1. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014; 384:766–781.2. Segula D. Complications of obesity in adults: a short review of the literature. Malawi Med J. 2014; 26:20–24.3. Grand View Research. Obesity treatment market size & forecast, by drugs (combination drugs, appetite suppressants, malabsorption & satiety drugs), by surgery & devices (adjustable gastric banding, Rouxen-Y gastric bypass, sleeve gastrectomy, biliopancreatic diversion with duodenal switch, endoscopic procedures) and trend analysis to 2024 [Internet]. San Francisco (CA): Grand View Research;c2016. [updated 2016 Sep; cited 2018 Sep 20]. Available from: https://www.grandviewresearch.com/industry-analysis/obesity-treatment-devices-and-therapeutics-market.4. Daniel S, Soleymani T, Garvey WT. A complications-based clinical staging of obesity to guide treatment modality and intensity. Curr Opin Endocrinol Diabetes Obes. 2013; 20:377–388.
Article5. Nguyen NT, Vu S, Kim E, Bodunova N, Phelan MJ. Trends in utilization of bariatric surgery, 2009-2012. Surg Endosc. 2016; 30:2723–2727.
Article6. Vargas EJ, Rizk M, Bazerbachi F, Abu Dayyeh BK. Medical devices for obesity treatment: endoscopic bariatric therapies. Med Clin North Am. 2018; 102:149–163.7. Sullivan S, Edmundowicz SA, Thompson CC. Endoscopic bariatric and metabolic therapies: new and emerging technologies. Gastroenterology. 2017; 152:1791–1801.
Article8. Kim SH, Chun HJ, Choi HS, Kim ES, Keum B, Jeen YT. Current status of intragastric balloon for obesity treatment. World J Gastroenterol. 2016; 22:5495–5504.
Article9. Gleysteen JJ. A history of intragastric balloons. Surg Obes Relat Dis. 2016; 12:430–435.
Article10. Nieben OG, Harboe H. Intragastric balloon as an artificial bezoar for treatment of obesity. Lancet. 1982; 1:198–199.11. Gomez V, Woodman G, Abu Dayyeh BK. Delayed gastric emptying as a proposed mechanism of action during intragastric balloon therapy: results of a prospective study. Obesity (Silver Spring). 2016; 24:1849–1853.12. Swidnicka-Siergiejko A, Wróblewski E, Andrzej D. Endoscopic treatment of obesity. Can J Gastroenterol. 2011; 25:627–633.
Article13. Kentish SJ, Page AJ. The role of gastrointestinal vagal afferent fibres in obesity. J Physiol. 2015; 593:775–786.
Article14. Brooks J, Srivastava ED, Mathus-Vliegen EM. One-year adjustable intragastric balloons: results in 73 consecutive patients in the U.K. Obes Surg. 2014; 24:813–819.
Article15. Machytka E, Gaur S, Chuttani R, et al. Elipse, the first procedureless gastric balloon for weight loss: a prospective, observational, open-label, multicenter study. Endoscopy. 2017; 49:154–160.
Article16. Lecumberri E, Krekshi W, Matía P, et al. Effectiveness and safety of airfilled balloon Heliosphere BAG® in 82 consecutive obese patients. Obes Surg. 2011; 21:1508–1512.
Article17. Almeghaiseeb ES, Ashraf MF, Alamro RA, Almasoud AO, Alrobayan AA. Efficacy of intragastric balloon on weight reduction: Saudi perspective. World J Clin Cases. 2017; 5:140–147.
Article18. Żurawiński W, Sokołowski D, Krupa-Kotara K, Czech E, Sosada K. Evaluation of the results of treatment of morbid obesity by the endoscopic intragastric balloon implantation method. Wideochir Inne Tech Maloinwazyjne. 2017; 12:37–48.
Article19. Keren D, Rainis T. Intragastric balloons for overweight populations-1 year post removal. Obes Surg. 2018; 28:2368–2373.20. Gaggiotti G, Tack J, Garrido AB Jr, Palau M, Cappelluti G, Di Matteo F. Adjustable totally implantable intragastric prosthesis (ATIIP)-Endogast for treatment of morbid obesity: one-year follow-up of a multicenter prospective clinical survey. Obes Surg. 2007; 17:949–956.21. Carvalho GL, Barros CB, Okazaki M, et al. An improved intragastric balloon procedure using a new balloon: preliminary analysis of safety and efficiency. Obes Surg. 2009; 19:237–242.
Article22. Lopasso FP, Sakai P, Gazi BM, et al. A pilot study to evaluate the safety, tolerance, and efficacy of a novel stationary antral balloon (SAB) for obesity. J Clin Gastroenterol. 2008; 42:48–53.
Article23. Martin CK, Bellanger DE, Rau KK, Coulon S, Greenway FL. Safety of the Ullorex oral intragastric balloon for the treatment of obesity. J Diabetes Sci Technol. 2007; 1:574–581.
Article24. Ponce J, Woodman G, Swain J, et al. The REDUCE pivotal trial: a prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis. 2015; 11:874–881.
Article25. US Food and Drug Administration. Summary of safety and effectiveness data (SSED): ORBERA™ intragastric balloon system [Internet]. Silver Spring (MD): FDA;c2015. [updated 2015 Aug 5; cited 2018 Sep 20]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140008b.pdf.26. US Food and Drug Administration. Summary of safety and effectiveness data (SSED): Obalon balloon system [Internet]. Silver Spring (MD): FDA;c2016. [updated 2016 Sep 8; cited 2018 Sep 20]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160001b.pdf.27. Borges AC, Almeida PC, Furlani SMT, Cury MS, Gaur S. Intragastric balloons in high-risk obese patients in a Brazilian center: initial experience. Rev Col Bras Cir. 2018; 45:e1448.
Article28. Machytka E, Klvana P, Kornbluth A, et al. Adjustable intragastric balloons: a 12-month pilot trial in endoscopic weight loss management. Obes Surg. 2011; 21:1499–1507.
Article29. Russo T, Aprea G, Formisano C, et al. BioEnterics intragastric balloon (BIB) versus spatz adjustable balloon system (ABS): our experience in the elderly. Int J Surg. 2017; 38:138–140.
Article30. Daniel F, Abou Fadel C, Houmani Z, Salti N. Spatz 3 adjustable intragastric balloon: long-term safety concerns. Obes Surg. 2016; 26:159–160.
Article31. Bazerbachi F, Vargas Valls EJ, Abu Dayyeh BK. Recent clinical results of endoscopic bariatric therapies as an obesity intervention. Clin Endosc. 2017; 50:42–50.
Article32. Alsabah S, Al Haddad E, Ekrouf S, Almulla A, Al-Subaie S, Al Kendari M. The safety and efficacy of the procedureless intragastric balloon. Surg Obes Relat Dis. 2018; 14:311–317.
Article33. Angrisani L, Santonicola A, Vitiello A, Belfiore MP, Belfiore G, Iovino P. Elipse balloon: the pitfalls of excessive simplicity. Obes Surg. 2018; 28:1419–1421.
Article34. Trande P, Mussetto A, Mirante VG, et al. Efficacy, tolerance and safety of new intragastric air-filled balloon (Heliosphere BAG) for obesity: the experience of 17 cases. Obes Surg. 2010; 20:1227–1230.
Article35. Caglar E, Dobrucali A, Bal K. Gastric balloon to treat obesity: filled with air or fluid? Dig Endosc. 2013; 25:502–507.
Article36. De Castro ML, Morales MJ, Del Campo V, et al. Efficacy, safety, and tolerance of two types of intragastric balloons placed in obese subjects: a double-blind comparative study. Obes Surg. 2010; 20:1642–1646.
Article37. Giardiello C, Borrelli A, Silvestri E, Antognozzi V, Iodice G, Lorenzo M. Air-filled vs water-filled intragastric balloon: a prospective randomized study. Obes Surg. 2012; 22:1916–1919.
Article38. Drozdowski R, Wyleżoł M, Frączek M, Hevelke P, Giaro M, Sobanski P. Small bowel necrosis as a consequence of spontaneous deflation and migration of an air-filled intragastric balloon - a potentially life-threatening complication. Wideochir Inne Tech Maloinwazyjne. 2014; 9:292–296.
Article39. Bužga M, Evžen M, Pavel K, et al. Effects of the intragastric balloon MedSil on weight loss, fat tissue, lipid metabolism, and hormones involved in energy balance. Obes Surg. 2014; 24:909–915.
Article40. Buzga M, Kupka T, Siroky M, et al. Short-term outcomes of the new intragastric balloon End-Ball® for treatment of obesity. Wideochir Inne Tech Maloinwazyjne. 2016; 11:229–235.
Article41. Pezzo CT, de Souza TF, Fenero V, Suano-Souza FI, Grecco E, Sarni ROS. Efficacy and safety of intragastric balloon in the treatment of obesity in adolescent females. Nutrire. 2017; 42:26.
Article42. Neto MG, Silva LB, Grecco E, et al. Brazilian intragastric balloon consensus statement (BIBC): practical guidelines based on experience of over 40,000 cases. Surg Obes Relat Dis. 2018; 14:151–159.
Article43. Do TN, Ho KY, Phee SJ. A magnetic soft endoscopic capsule-inflated intragastric balloon for weight management. Sci Rep. 2016; 6:39486.
Article44. Ghoz HM, Acosta A, Topazian M, Camilleri M, Gostout C, Dayyeh BKA. A pre-clinical animal study of combined intragastric balloon and duodenal-jejunal bypass sleeve for obesity and metabolic disease. Gastroenterology. 2016; 150(4 Suppl 1):S231–S232.45. Choi HS, Chun HJ. Recent trends in endoscopic bariatric therapies. Clin Endosc. 2017; 50:11–16.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Clinical and Metabolic Effects of Intragastric Balloon on Morbid Obesity and Its Related Comorbidities
- Gastric Ulceration and Bleeding with Hemodynamic Instability Caused by an Intragastric Balloon for Weight Loss
- The Effects of the Small Doses of Nondepolarzing Muscle Relaxants Administered Just Prior to Succinylcholine on Intragastric and Intraocular Pressures
- The Association of Postsurgical Gastritis with Duodenogastric Reflux in Patients with Billroth-II Gastrectomy
- Effects of CYP2C19 Polymorphism on Intragastric Acidity during Omeprazole Administration