Endocrinol Metab.
2018 Dec;33(4):466-472. 10.3803/EnM.2018.33.4.466.
Thyroid-Stimulating Hormone Reference Ranges in the First Trimester of Pregnancy in an Iodine-Sufficient Country
- Affiliations
-
- 1Department of Medicine, San Juan de Dios Hospital and University of Talca, Curico, Chile.
- 2Department of Endocrinology, Faculty of Medicine, Pontifical Catholic University of Chile, Santiago, Chile. lmosso@uc.cl
- 3Department of Public Health, Faculty of Medicine, Pontifical Catholic University of Chile, Santiago, Chile.
- 4School of Public Health, Faculty of Medicine, University of Chile, Santiago, Chile.
- 5Department of Family Medicine, Faculty of Medicine, Pontifical Catholic University of Chile, Santiago, Chile.
- 6Department of Pediatrics, Faculty of Medicine, Diego Portales University, Santiago, Chile.
- KMID: 2427442
- DOI: http://doi.org/10.3803/EnM.2018.33.4.466
Abstract
- BACKGROUND
Thyroid dysfunction is associated with negative neonatal and obstetric outcomes. Large differences in thyroid function reference intervals exist across different populations. These differences can be explained by population-specific factors, such as iodine status. Many countries in Latin America report iodine sufficiency, but relatively few countries have published up-to-date data on iodine levels and thyroid function in the overall population, and especially in pregnant women. We evaluated the iodine status of pregnant women in Chile and determined thyroid hormone reference ranges in this population.
METHODS
This was a prospective observational study of healthy Chilean women at their first prenatal visit before week 14. Thyroid-stimulating hormone (TSH), total thyroxine (T4), free T4, antithyroid peroxidase antibody (TPOAb), and iodine levels from spot urine samples were measured. Iodine status and the reference ranges for TSH were calculated.
RESULTS
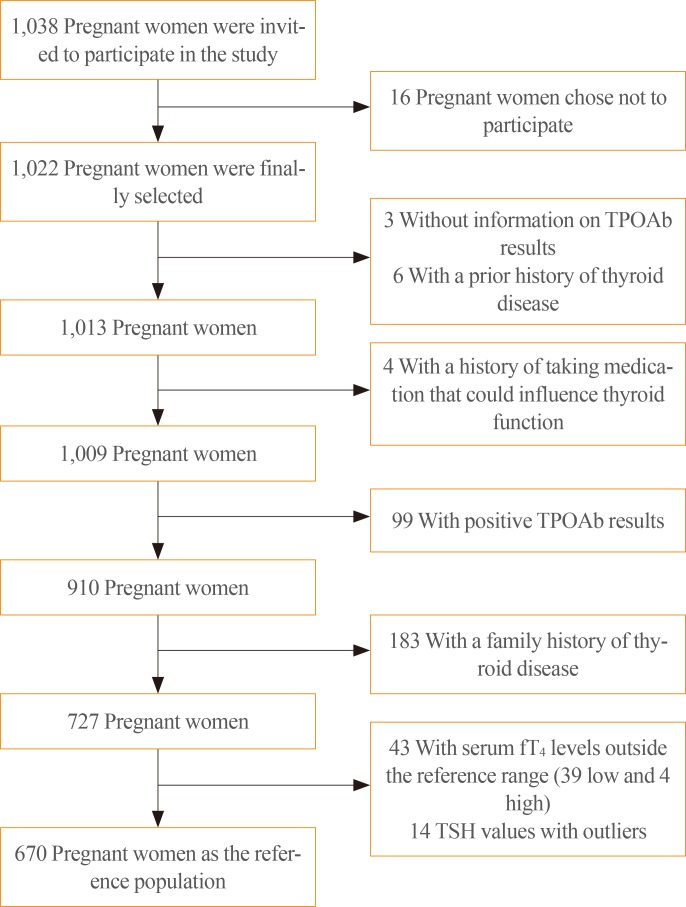
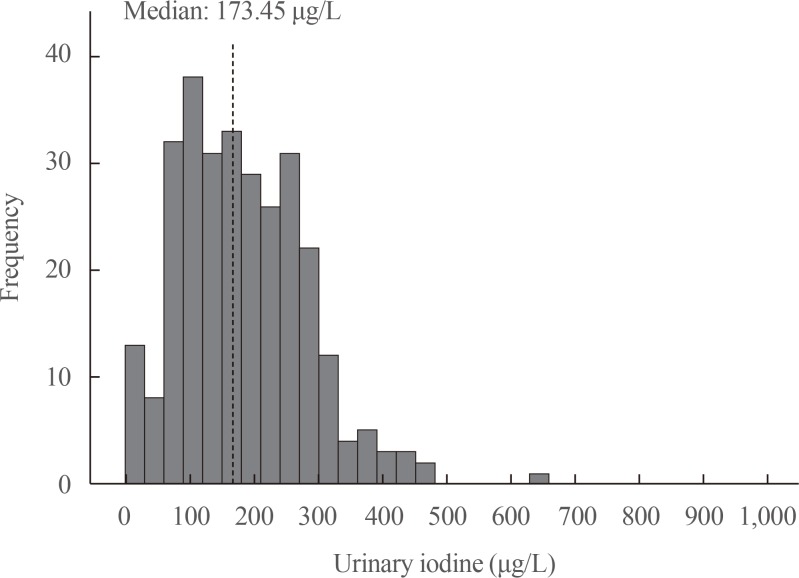
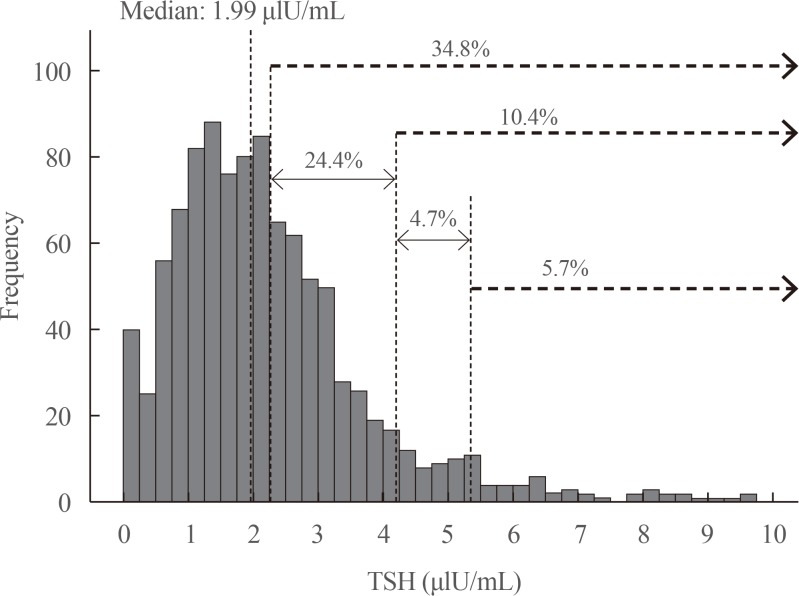
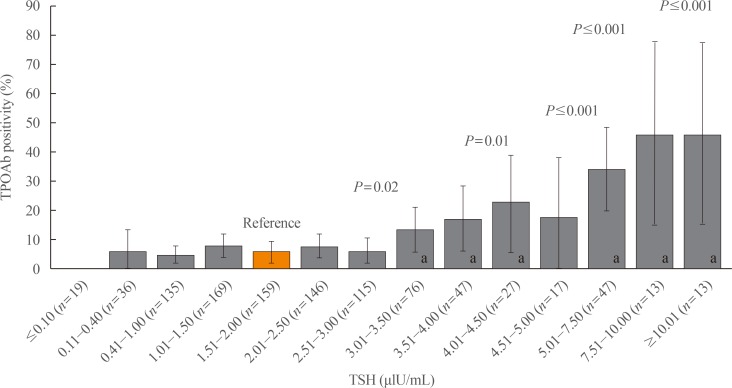
A total of 1,022 pregnant women in the first trimester were selected. Urinary iodine levels were measured in 302 randomly-selected women. The median urinary iodine concentration was 173.45 µg/L (interquartile range, 108.11 to 249.35).The reference ranges of TSH were calculated in 670 patients selected according to the National Academy of Clinical Biochemistry guidelines. The median TSH level was 1.88 µIU/mL (2.5th percentile: 0.13 to 97.5th percentile: 5.37). Using the reference range in the 1,022 women, the prevalence of clinical hypothyroidism was 1.76%, and that of subclinical hypothyroidism was 3.92%. TPOAb positivity was more common in women with TSH levels above 3.5 µIU/mL.
CONCLUSION
We found adequate iodine intake and a right-shifted distribution of serum TSH levels in pregnant women in Chile. The prevalence of hypothyroidism in our sample of pregnant women was higher than has been described in the literature.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Thyroid-Stimulating Hormone Reference Ranges in Early Pregnancy: Possible Influence of Iodine Status
Tae Yong Kim
Endocrinol Metab. 2018;33(4):445-446. doi: 10.3803/EnM.2018.33.4.445.Response: Thyroid-Stimulating Hormone Reference Ranges in the First Trimester of Pregnancy in an Iodine-Sufficient Country (Endocrinol Metab 2018;33:466–72, Carmen Castillo et al.)
Lorena Mosso
Endocrinol Metab. 2019;34(2):213-214. doi: 10.3803/EnM.2019.34.2.213.Letter: Thyroid-Stimulating Hormone Reference Ranges in the First Trimester of Pregnancy in an Iodine-Sufficient Country (Endocrinol Metab 2018;33:466-72, Carmen Castillo et al.)
Hyemi Kwon
Endocrinol Metab. 2019;34(1):93-94. doi: 10.3803/EnM.2019.34.1.93.
Reference
-
1. Abalovich M, Amino N, Barbour LA, Cobin RH, De Groot LJ, Glinoer D, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007; 92(8 Suppl):S1–S47. PMID: 17948378.
Article2. Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011; 21:1081–1125. PMID: 21787128.
Article3. Qaseem A, Snow V, Owens DK, Shekelle P. The development of clinical practice guidelines and guidance statements of the American College of Physicians: summary of methods. Ann Intern Med. 2010; 153:194–199. PMID: 20679562.
Article4. Orito Y, Oku H, Kubota S, Amino N, Shimogaki K, Hata M, et al. Thyroid function in early pregnancy in Japanese healthy women: relation to urinary iodine excretion, emesis, and fetal and child development. J Clin Endocrinol Metab. 2009; 94:1683–1688. PMID: 19258403.
Article5. Yan YQ, Dong ZL, Dong L, Wang FR, Yang XM, Jin XY, et al. Trimester- and method-specific reference intervals for thyroid tests in pregnant Chinese women: methodology, euthyroid definition and iodine status can influence the setting of reference intervals. Clin Endocrinol (Oxf). 2011; 74:262–269. PMID: 21044115.
Article6. Marwaha RK, Chopra S, Gopalakrishnan S, Sharma B, Kanwar RS, Sastry A, et al. Establishment of reference range for thyroid hormones in normal pregnant Indian women. BJOG. 2008; 115:602–606. PMID: 18333941.
Article7. Moon HW, Chung HJ, Park CM, Hur M, Yun YM. Establishment of trimester-specific reference intervals for thyroid hormones in Korean pregnant women. Ann Lab Med. 2015; 35:198–204. PMID: 25729721.
Article8. Mosso L, Martinez A, Rojas MP, Margozzini P, Solari S, Lyng T, et al. Frequency of subclinical thyroid problems among women during the first trimester of pregnancy. Rev Med Chil. 2012; 140:1401–1408. PMID: 23677185.9. Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003; 13:3–126. PMID: 12625976.10. De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97:2543–2565. PMID: 22869843.
Article11. Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. 2014; 3:76–94. PMID: 25114871.
Article12. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017; 27:315–389. PMID: 28056690.
Article13. Li C, Shan Z, Mao J, Wang W, Xie X, Zhou W, et al. Assessment of thyroid function during first-trimester pregnancy: what is the rational upper limit of serum TSH during the first trimester in Chinese pregnant women? J Clin Endocrinol Metab. 2014; 99:73–79. PMID: 24276458.
Article14. Shi X, Han C, Li C, Mao J, Wang W, Xie X, et al. Optimal and safe upper limits of iodine intake for early pregnancy in iodine-sufficient regions: a cross-sectional study of 7190 pregnant women in China. J Clin Endocrinol Metab. 2015; 100:1630–1638. PMID: 25629356.
Article15. Council on Environmental Health. Rogan WJ, Paulson JA, Baum C, Brock-Utne AC, Brumberg HL, et al. Iodine deficiency, pollutant chemicals, and the thyroid: new information on an old problem. Pediatrics. 2014; 133:1163–1166. PMID: 24864180.16. Kim WG, Kim WB, Woo G, Kim H, Cho Y, Kim TY, et al. Thyroid stimulating hormone reference range and prevalence of thyroid dysfunction in the Korean population: Korea National Health and Nutrition Examination Survey 2013 to 2015. Endocrinol Metab (Seoul). 2017; 32:106–114. PMID: 28116874.
Article17. Muzzo S, Burgueno M, Carvajal F, Biolley E, Avendano M, Vargas S, et al. Iodine nutrition in school children of four census areas of Chile. Rev Med Chil. 1997; 125:1299–1304. PMID: 9609050.18. EUthyroid. Laboratory method and statistical analysis for cross-lab comparison [Internet]. Vienna: Biolution GmbH;c2015. cited 2018 Nov 5. Available from: http://euthyroid.eu/download/EUthyroid_iodinemap_textEN.pdf.19. World Health Organization. Assessment of iodine deficiency disorders and monitoring their elimination. 3rd ed. Geneva: World Health Organization;2007.20. Muzzo S, Leiva L, Ramirez I, Carvajal F, Biolley E. Iodine nutrition status in school age children of an area of high iodine intake (Calama) compared with an area of normal intake (Punta Arenas). Rev Chil Nutr. 2005; 32:28–35.21. Mioto VCB, Monteiro ACCNG, de Camargo RYA, Borel AR, Catarino RM, Kobayashi S, et al. High prevalence of iodine deficiency in pregnant women living in adequate iodine area. Endocr Connect. 2018; 7:762–767. PMID: 29700098.
Article22. Medici M, Korevaar TI, Visser WE, Visser TJ, Peeters RP. Thyroid function in pregnancy: what is normal? Clin Chem. 2015; 61:704–713. PMID: 25829408.
Article23. Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: trends and associations across trimesters in iodine sufficiency. Thyroid. 2004; 14:1084–1090. PMID: 15650363.
Article24. Quinn FA, Gridasov GN, Vdovenko SA, Krasnova NA, Vodopianova NV, Epiphanova MA, et al. Prevalence of abnormal thyroid stimulating hormone and thyroid peroxidase antibody-positive results in a population of pregnant women in the Samara region of the Russian Federation. Clin Chem Lab Med. 2005; 43:1223–1226. PMID: 16232090.
Article25. Stricker R, Echenard M, Eberhart R, Chevailler MC, Perez V, Quinn FA, et al. Evaluation of maternal thyroid function during pregnancy: the importance of using gestational age-specific reference intervals. Eur J Endocrinol. 2007; 157:509–514. PMID: 17893266.
Article26. Korevaar TIM. The upper limit for TSH during pregnancy: why we should stop using fixed limits of 2.5 or 3.0 mU/l. Thyroid Res. 2018; 11:5. PMID: 29942352.
Article27. Mosso L, Martinez A, Rojas MP, Latorre G, Margozzini P, Lyng T, et al. Early pregnancy thyroid hormone reference ranges in Chilean women: the influence of body mass index. Clin Endocrinol (Oxf). 2016; 85:942–948. PMID: 27260560.
Article28. Spencer CA, Hollowell JG, Kazarosyan M, Braverman LE. National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab. 2007; 92:4236–4240. PMID: 17684054.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter: Thyroid-Stimulating Hormone Reference Ranges in the First Trimester of Pregnancy in an Iodine-Sufficient Country (Endocrinol Metab 2018;33:466-72, Carmen Castillo et al.)
- Response: Thyroid-Stimulating Hormone Reference Ranges in the First Trimester of Pregnancy in an Iodine-Sufficient Country (Endocrinol Metab 2018;33:466–72, Carmen Castillo et al.)
- Thyroid-Stimulating Hormone Reference Ranges in Early Pregnancy: Possible Influence of Iodine Status
- Reference intervals of thyroid hormones during pregnancy in Korea, an iodine-replete area
- TSH and Free T4 Concentrations in Korean Pregnant Women