Korean J Radiol.
2017 Jun;18(3):510-518. 10.3348/kjr.2017.18.3.510.
Assessment of Cervical Cancer with a Parameter-Free Intravoxel Incoherent Motion Imaging Algorithm
- Affiliations
-
- 1Institute of Diagnostic and Interventional Radiology, University Hospital of Zurich, Zurich 8091, Switzerland. anton.becker@usz.ch
- 2Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, China.
- KMID: 2427306
- DOI: http://doi.org/10.3348/kjr.2017.18.3.510
Abstract
OBJECTIVE
To evaluate the feasibility of a parameter-free intravoxel incoherent motion (IVIM) approach in cervical cancer, to assess the optimal b-value threshold, and to preliminarily examine differences in the derived perfusion and diffusion parameters for different histological cancer types.
MATERIALS AND METHODS
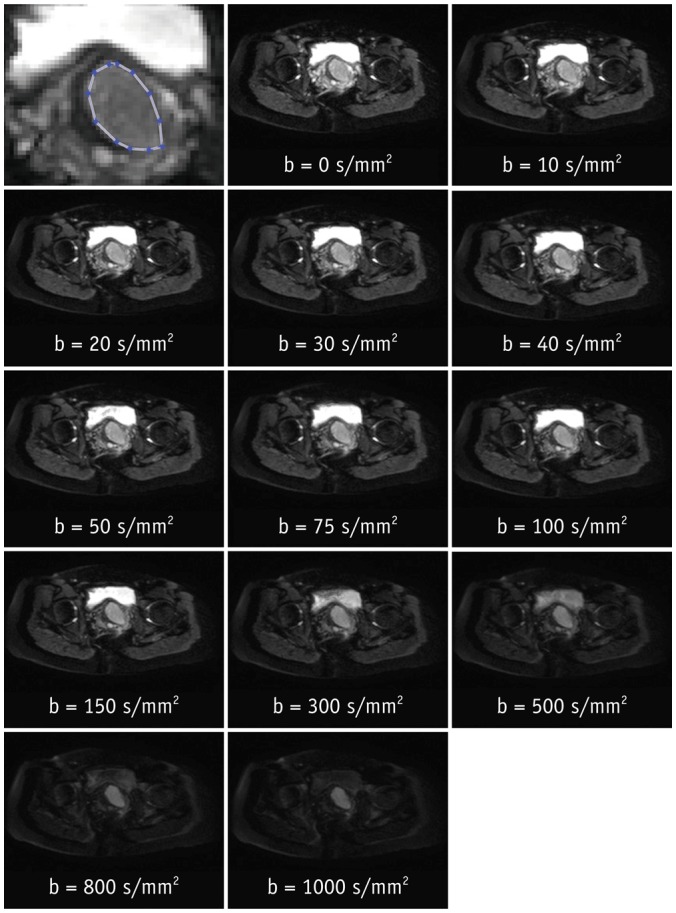
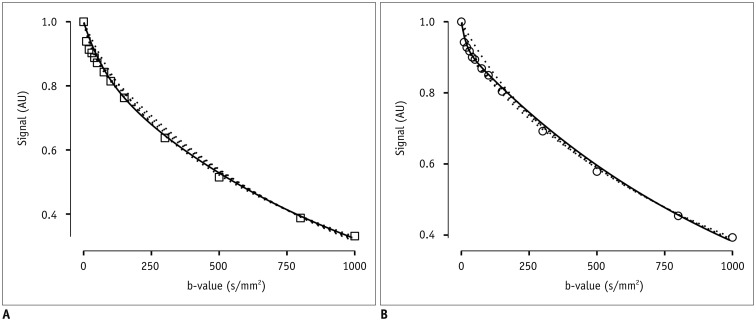
After Institutional Review Board approval, 19 female patients (mean age, 54 years; age range, 37-78 years) gave consent and were enrolled in this prospective magnetic resonance imaging study. Clinical staging and biopsy results were obtained. Echo-planar diffusion weighted sequences at 13 b-values were acquired at 3 tesla field strength. Single-sliced region-of-interest IVIM analysis with adaptive b-value thresholds was applied to each tumor, yielding the optimal fit and the optimal parameters for pseudodiffusion (D*), perfusion fraction (F(p)) and diffusion coefficient (D). Monoexponential apparent diffusion coefficient (ADC) was calculated for comparison with D.
RESULTS
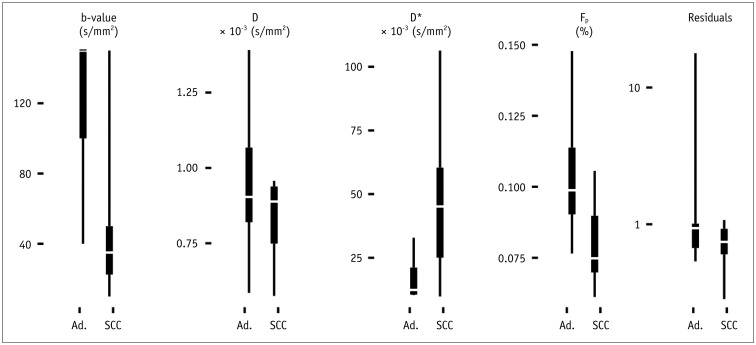
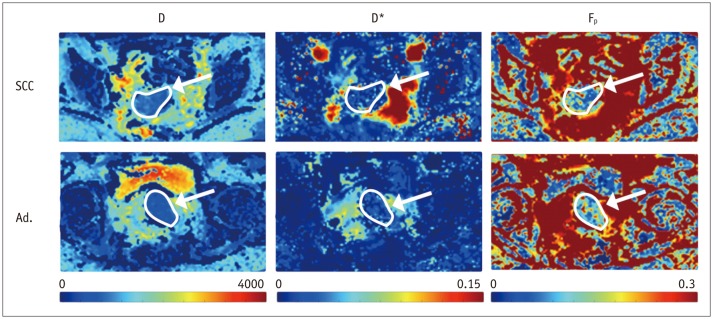
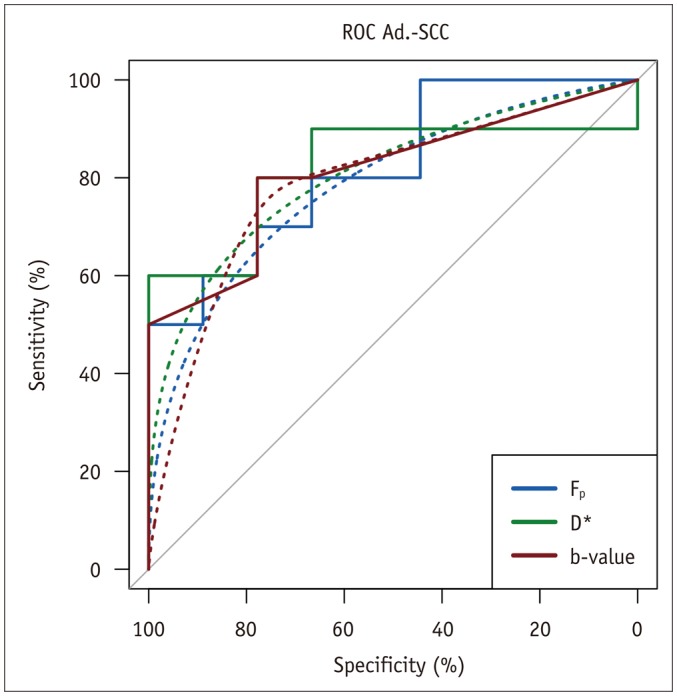
Biopsy revealed squamous cell carcinoma in 10 patients and adenocarcinoma in 9. The b-value threshold (median [interquartile range]) depended on the histological type and was 35 (22.5-50) s/mm² in squamous cell carcinoma and 150 (100-150) s/mm² in adenocarcinoma (p < 0.05). Comparing squamous cell vs. adenocarcinoma, D* (45.1 [25.1-60.4] × 10⻳ mm²/s vs. 12.4 [10.5-21.2] × 10⻳ mm²/s) and F(p) (7.5% [7.0-9.0%] vs. 9.9% [9.0-11.4%]) differed significantly between the subtypes (p < 0.02), whereas D did not (0.89 [0.75-0.94] × 10⻳ mm²/s vs. 0.90 [0.82-0.97] × 10⻳ mm²/s, p = 0.27). The residuals did not differ (0.74 [0.60-0.92] vs. 0.94 [0.67-1.01], p = 0.32). The ADC systematically underestimated the magnitude of diffusion restriction compared to D (p < 0.001).
CONCLUSION
The parameter-free IVIM approach is feasible in cervical cancer. The b-value threshold and perfusion-related parameters depend on the tumor histology type.
MeSH Terms
-
Adenocarcinoma/*diagnosis/diagnostic imaging/pathology
Adult
Aged
*Algorithms
Area Under Curve
Carcinoma, Squamous Cell/*diagnosis/diagnostic imaging/pathology
Diffusion Magnetic Resonance Imaging
Female
Humans
Middle Aged
Neoplasm Staging
Prospective Studies
ROC Curve
Uterine Cervical Neoplasms/*diagnosis/diagnostic imaging/pathology
Figure
Cited by 2 articles
-
B-Value Optimization in the Estimation of Intravoxel Incoherent Motion Parameters in Patients with Cervical Cancer
Jose Angelo Udal Perucho, Hing Chiu Charles Chang, Varut Vardhanabhuti, Mandi Wang, Anton Sebastian Becker, Moritz Christoph Wurnig, Elaine Yuen Phin Lee
Korean J Radiol. 2020;21(2):218-227. doi: 10.3348/kjr.2019.0232.Age of Data in Contemporary Research Articles Published in Representative General Radiology Journals
Ji Hun Kang, Dong Hwan Kim, Seong Ho Park, Jung Hwan Baek
Korean J Radiol. 2018;19(6):1172-1178. doi: 10.3348/kjr.2018.19.6.1172.
Reference
-
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108. PMID: 25651787.
Article2. Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014; 383:524–532. PMID: 24192252.
Article3. Wakefield JC, Downey K, Kyriazi S, deSouza NM. New MR techniques in gynecologic cancer. AJR Am J Roentgenol. 2013; 200:249–260. PMID: 23345344.
Article4. Koh WJ, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Chan J, et al. Cervical cancer. J Natl Compr Canc Netw. 2013; 11:320–343. PMID: 23486458.
Article5. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988; 168:497–505. PMID: 3393671.
Article6. Zhou Y, Liu J, Liu C, Jia J, Li N, Xie L, et al. Intravoxel incoherent motion diffusion weighted MRI of cervical cancer-correlated with tumor differentiation and perfusion. Magn Reson Imaging. 2016; 34:1050–1056. PMID: 27133158.7. Lee EY, Yu X, Chu MM, Ngan HY, Siu SW, Soong IS, et al. Perfusion and diffusion characteristics of cervical cancer based on intraxovel incoherent motion MR imaging-a pilot study. Eur Radiol. 2014; 24:1506–1513. PMID: 24744198.
Article8. Lorenz CH, Pickens DR 3rd, Puffer DB, Price RR. Magnetic resonance diffusion/perfusion phantom experiments. Magn Reson Med. 1991; 19:254–260. PMID: 1881312.
Article9. Alison M, Chalouhi GE, Autret G, Balvay D, Thiam R, Salomon LJ, et al. Use of intravoxel incoherent motion MR imaging to assess placental perfusion in a murine model of placental insufficiency. Invest Radiol. 2013; 48:17–23. PMID: 23192161.
Article10. Wurnig MC, Donati OF, Ulbrich E, Filli L, Kenkel D, Thoeny HC, et al. Systematic analysis of the intravoxel incoherent motion threshold separating perfusion and diffusion effects: proposal of a standardized algorithm. Magn Reson Med. 2015; 74:1414–1422. PMID: 25360990.
Article11. Stieb S, Boss A, Wurnig MC, Özbay PS, Weiss T, Guckenberger M, et al. Non-parametric intravoxel incoherent motion analysis in patients with intracranial lesions: test-retest reliability and correlation with arterial spin labeling. Neuroimage Clin. 2016; 11:780–788. PMID: 27354956.
Article12. Koh DM, Collins DJ, Orton MR. Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. AJR Am J Roentgenol. 2011; 196:1351–1361. PMID: 21606299.
Article13. Landis JR, King TS, Choi JW, Chinchilli VM, Koch GG. Measures of agreement and concordance with clinical research applications. Stat Biopharm Res. 2012; 3:185–209.
Article14. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol). 1995; 57:289–230.
Article15. Wickham H. Ggplot2: elegant graphics for data analysis. New York: Springer Science & Business Media;2009.16. Winfield JM, Orton MR, Collins DJ, Ind TE, Attygalle A, Hazell S, et al. Separation of type and grade in cervical tumours using non-mono-exponential models of diffusion-weighted MRI. Eur Radiol. 2017; 27:627–636. PMID: 27221560.
Article17. Lee EY, Hui ES, Chan KK, Tse KY, Kwong WK, Chang TY, et al. Relationship between intravoxel incoherent motion diffusion-weighted MRI and dynamic contrast-enhanced MRI in tissue perfusion of cervical cancers. J Magn Reson Imaging. 2015; 42:454–459. PMID: 25413245.
Article18. Fujimoto J, Sakaguchi H, Hirose R, Ichigo S, Tamaya T. Expression of vascular endothelial growth factor (VEGF) and its mRNA in uterine cervical cancers. Br J Cancer. 1999; 80:827–833. PMID: 10360662.
Article19. Saijo Y, Furumoto H, Yoshida K, Nishimura M, Irahara M. Clinical significance of vascular endothelial growth factor expression and microvessel density in invasive cervical cancer. J Med Invest. 2015; 62:154–160. PMID: 26399340.
Article20. Eberhardt C, Wurnig MC, Wirsching A, Rossi C, Rottmar M, Özbay PS, et al. Intravoxel incoherent motion analysis of abdominal organs: computation of reference parameters in a large cohort of C57Bl/6 mice and correlation to microvessel density. MAGMA. 2016; 29:751–763. PMID: 27094553.
Article21. Lee HJ, Rha SY, Chung YE, Shim HS, Kim YJ, Hur J, et al. Tumor perfusion-related parameter of diffusion-weighted magnetic resonance imaging: correlation with histological microvessel density. Magn Reson Med. 2014; 71:1554–1558. PMID: 23798038.
Article22. Shimada M, Nishimura R, Nogawa T, Hatae M, Takehara K, Yamada H, et al. Comparison of the outcome between cervical adenocarcinoma and squamous cell carcinoma patients with adjuvant radiotherapy following radical surgery: SGSG/TGCU Intergroup Surveillance. Mol Clin Oncol. 2013; 1:780–784. PMID: 24649246.
Article23. Katanyoo K, Sanguanrungsirikul S, Manusirivithaya S. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma in locally advanced cervical cancer. Gynecol Oncol. 2012; 125:292–296. PMID: 22293041.
Article24. Galic V, Herzog TJ, Lewin SN, Neugut AI, Burke WM, Lu YS, et al. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol Oncol. 2012; 125:287–291. PMID: 22266551.
Article25. Lin G, Lai CH, Tsai SY, Lin YC, Huang YT, Wu RC, et al. 1H MR spectroscopy in cervical carcinoma using external phase array body coil at 3.0 Tesla: prediction of poor prognostic human papillomavirus genotypes. J Magn Reson Imaging. 2017; 45:899–907. PMID: 27434095.
Article26. McVeigh PZ, Syed AM, Milosevic M, Fyles A, Haider MA. Diffusion-weighted MRI in cervical cancer. Eur Radiol. 2008; 18:1058–1064. PMID: 18193428.
Article27. Lemke A, Stieltjes B, Schad LR, Laun FB. Toward an optimal distribution of b values for intravoxel incoherent motion imaging. Magn Reson Imaging. 2011; 29:766–776. PMID: 21549538.
Article28. Lemke A, Laun FB, Klauss M, Re TJ, Simon D, Delorme S, et al. Differentiation of pancreas carcinoma from healthy pancreatic tissue using multiple b-values: comparison of apparent diffusion coefficient and intravoxel incoherent motion derived parameters. Invest Radiol. 2009; 44:769–775. PMID: 19838121.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- B-Value Optimization in the Estimation of Intravoxel Incoherent Motion Parameters in Patients with Cervical Cancer
- Longitudinal Assessment of Intravoxel Incoherent Motion Diffusion Weighted Imaging in Evaluating the Radio-sensitivity of Nasopharyngeal Carcinoma Treated with Intensity-Modulated Radiation Therapy
- Comparative Study between ZOOMit and Conventional Intravoxel Incoherent Motion MRI for Assessing Parotid Gland Abnormalities in Patients with Early- or Mid-Stage Sjögren’s Syndrome
- Intravoxel Incoherent Motion MR Imaging in the Head and Neck: Correlation with Dynamic Contrast-Enhanced MR Imaging and Diffusion-Weighted Imaging
- RE: Distinguishing between Renal Cell Carcinoma and Fat Poor Angiomyolipoma in Diffusion-Weighted Imaging