Korean J Radiol.
2017 Jun;18(3):413-426. 10.3348/kjr.2017.18.3.413.
Hepatic Lesions that Mimic Metastasis on Radiological Imaging during Chemotherapy for Gastrointestinal Malignancy: Recent Updates
- Affiliations
-
- 1Department of Radiology, Anam Hospital, Korea University College of Medicine, Seoul 02841, Korea. radiolbj226@gmail.com
- 2Department of Internal Medicine, Anam Hospital, Korea University College of Medicine, Seoul 02841, Korea.
- KMID: 2427297
- DOI: http://doi.org/10.3348/kjr.2017.18.3.413
Abstract
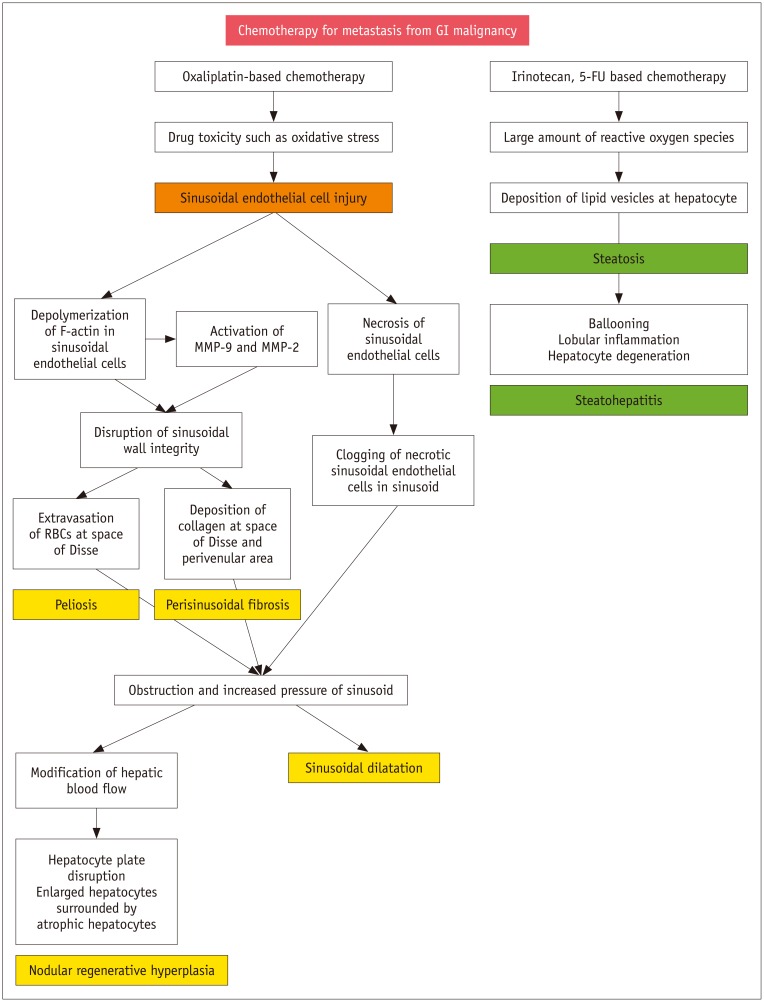
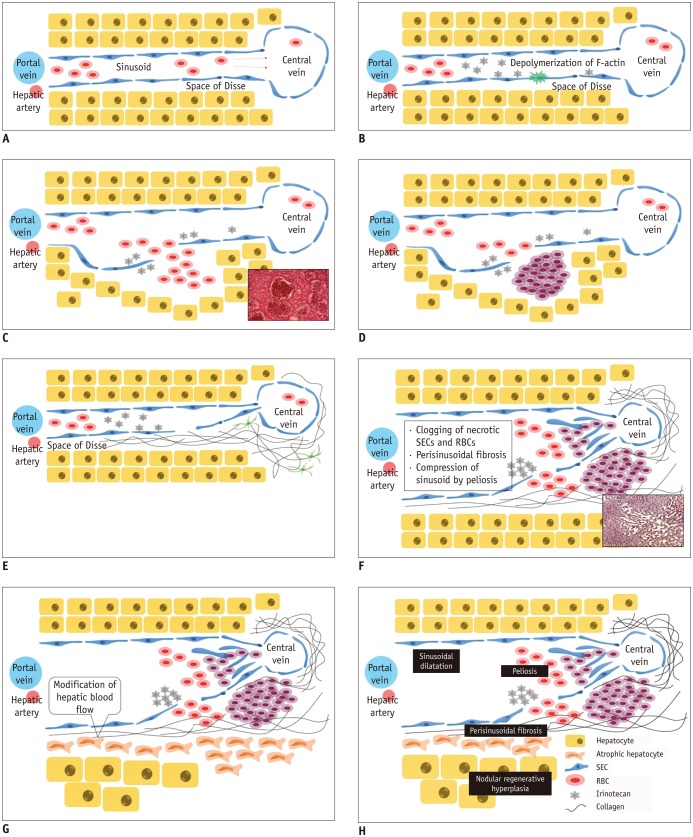
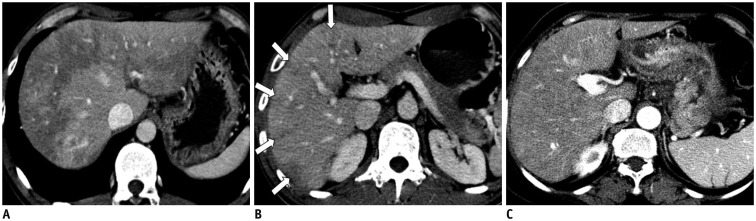
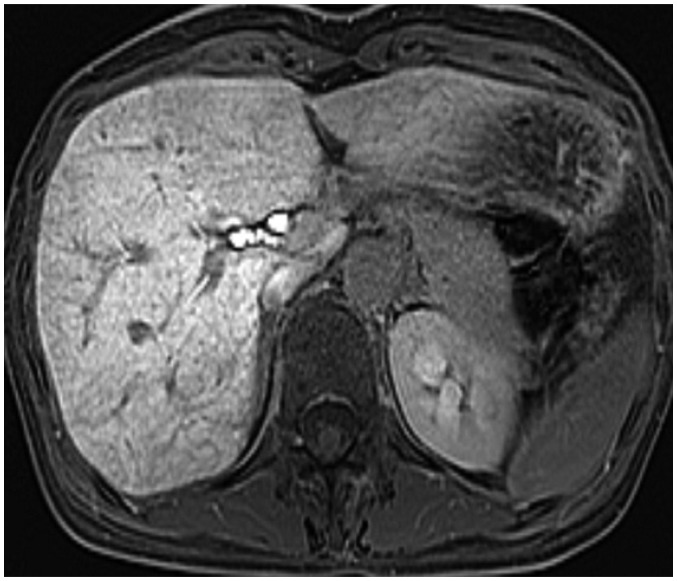
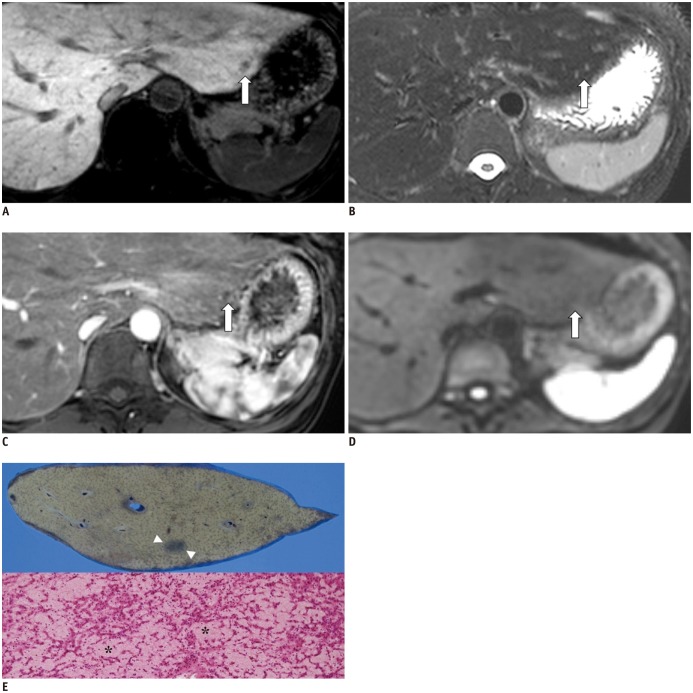
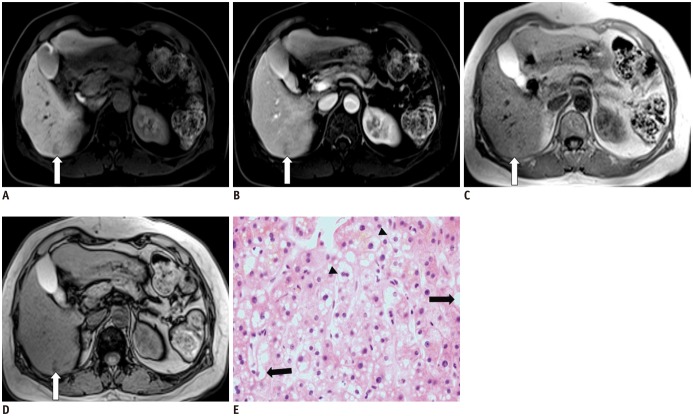
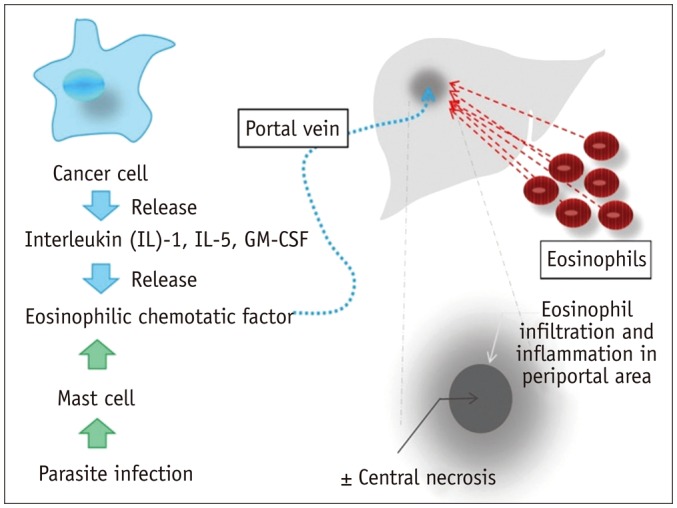
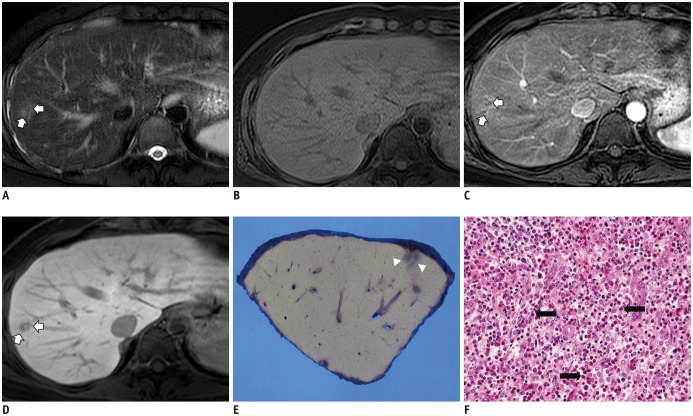
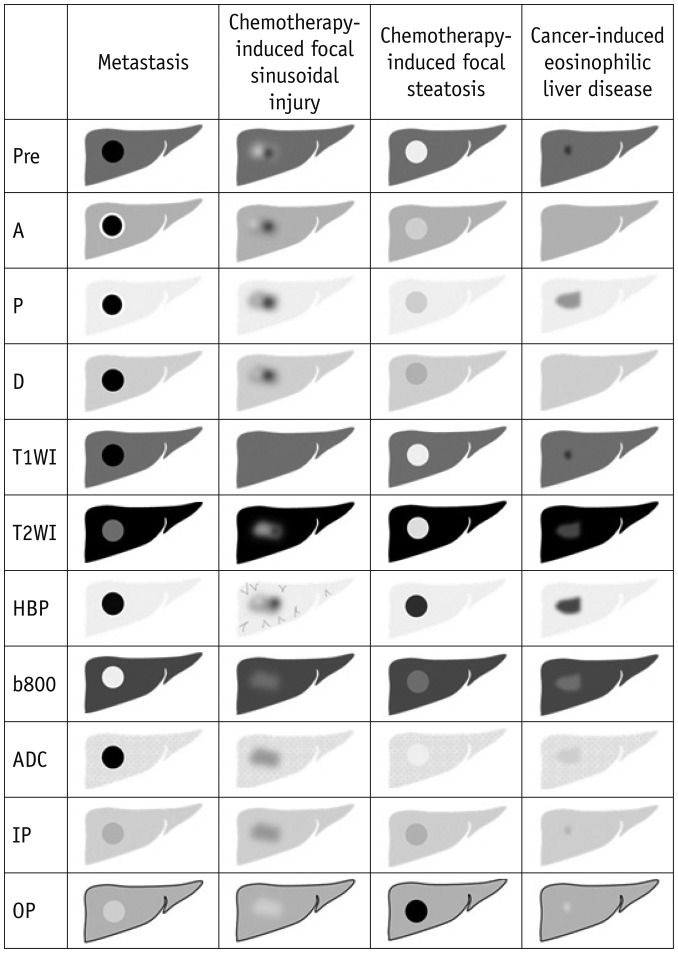
- During chemotherapy in patients with gastrointestinal malignancy, the hepatic lesions may occur as chemotherapy-induced lesions or tumor-associated lesions, with exceptions for infectious conditions and other incidentalomas. Focal hepatic lesions arising from chemotherapy-induced hepatopathies (such as chemotherapy-induced sinusoidal injury and steatosis) and tumor-associated eosinophilic abscess should be considered a mimicker of metastasis in patients with gastrointestinal malignancy. Accumulating evidence suggests that chemotherapy for gastrointestinal malignancy in the liver has roles in both the therapeutic effects for hepatic metastasis and injury to the non-tumor bearing hepatic parenchyma. In this article, we reviewed the updated concept of chemotherapy-induced hepatopathies and tumor-associated eosinophilic abscess in the liver, focusing on the pathological and radiological findings. Awareness of the causative chemo-agent, pathophysiology, and characteristic imaging findings of these mimickers is critical for accurate diagnosis and avoidance of unnecessary exposure of the patient to invasive tissue-based diagnosis and operations.
Keyword
MeSH Terms
-
Abscess/diagnostic imaging/etiology
Antineoplastic Combined Chemotherapy Protocols/adverse effects/*therapeutic use
Chemical and Drug Induced Liver Injury/*diagnostic imaging/etiology/pathology
Fatty Liver/diagnostic imaging/etiology/pathology
Gastrointestinal Neoplasms/*drug therapy/pathology
Hepatic Veno-Occlusive Disease/diagnostic imaging/etiology/pathology
Humans
Liver Neoplasms/pathology/secondary
Magnetic Resonance Imaging
Tomography, X-Ray Computed
Figure
Reference
-
1. Han NY, Park BJ, Sung DJ, Kim MJ, Cho SB, Lee CH, et al. Chemotherapy-induced focal hepatopathy in patients with gastrointestinal malignancy: gadoxetic acid--enhanced and diffusion-weighted MR imaging with clinical-pathologic correlation. Radiology. 2014; 271:416–425. PMID: 24475862.
Article2. Zhou B, Yan S, Zheng S. Peliosis hepatis: a mimicker of hepatic tumors. Surg Pract. 2013; 7:124–126.
Article3. Arakawa Y, Shimada M, Utsunomya T, Imura S, Morine Y, Ikemoto T, et al. Oxaliplatin-related sinusoidal obstruction syndrome mimicking metastatic liver tumors. Hepatol Res. 2013; 43:685–689. PMID: 23730707.
Article4. Kim MJ, Kim HJ, Yi BH, Kim HK, Yun J, Kim SH, et al. A case of peliosis hepatis mimicking metastatic lesions of increasing size. Soonchunhyang Med Sci. 2012; 18:141–144.
Article5. Xiong WJ, Hu LJ, Jian YC, He Y, Zhou W, Guo XL, et al. Focal peliosis hepatis in a colon cancer patient resembling metastatic liver tumor. World J Gastroenterol. 2012; 18:5999–6002. PMID: 23139621.
Article6. Nam SJ, Cho JY, Lee HS, Choe G, Jang JJ, Yoon YS, et al. Chemotherapy-associated hepatopathy in Korean colorectal cancer liver metastasis patients: oxaliplatin-based chemotherapy and sinusoidal injury. Korean J Pathol. 2012; 46:22–29. PMID: 23109974.
Article7. Kim SH, Lee JM, Kim WH, Han JK, Lee JY, Choi BI. Focal peliosis hepatis as a mimicker of hepatic tumors: radiological-pathological correlation. J Comput Assist Tomogr. 2007; 31:79–85. PMID: 17259837.8. Choi JS, Kim MJ. Education and imaging: hepatobiliary and pancreatic: focal steatohepatitis mimicking a metastasis. J Gastroenterol Hepatol. 2011; 26:415. PMID: 21261737.9. Lee T, Lee YS, Yoon SY, Kim SJ, Bae YJ, Kwon HS, et al. Clinical characteristics that distinguish eosinophilic organ infiltration from metastatic nodule development in cancer patients with eosinophilia. World J Surg Oncol. 2012; 10:175. PMID: 22929225.
Article10. Lee MH, Kim SH, Kim H, Lee MW, Lee WJ. Differentiating focal eosinophilic infiltration from metastasis in the liver with gadoxetic acid-enhanced magnetic resonance imaging. Korean J Radiol. 2011; 12:439–449. PMID: 21852904.
Article11. Kim YK, Lee YH, Kim CS, Lee MW. Differentiating focal eosinophilic liver disease from hepatic metastases using unenhanced and gadoxetic acid-enhanced MRI. Abdom Imaging. 2011; 36:425–432. PMID: 21748468.
Article12. Zhang KK, Maybody M, Shah RP, Vakiani E, Getrajdman GI, Brody LA, et al. Asymptomatic liver abscesses mimicking metastases in patients after whipple surgery: infectious complications following percutaneous biopsy-a report of two cases. Case Reports Hepatol. 2012; 2012:817314. PMID: 25374711.
Article13. Torrisi JM, Schwartz LH, Gollub MJ, Ginsberg MS, Bosl GJ, Hricak H. CT findings of chemotherapy-induced toxicity: what radiologists need to know about the clinical and radiologic manifestations of chemotherapy toxicity. Radiology. 2011; 258:41–56. PMID: 21183492.
Article14. O'Reilly DA, Poston GJ. Colorectal liver metastases: current and future perspectives. Future Oncol. 2006; 2:525–531. PMID: 16922619.15. Sugihara K, Uetake H. Therapeutic strategies for hepatic metastasis of colorectal cancer: overview. J Hepatobiliary Pancreat Sci. 2012; 19:523–527. PMID: 22706522.
Article16. Alberts SR, Wagman LD. Chemotherapy for colorectal cancer liver metastases. Oncologist. 2008; 13:1063–1073. PMID: 18838438.
Article17. Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004; 15:460–466. PMID: 14998849.
Article18. Pawlik TM, Olino K, Gleisner AL, Torbenson M, Schulick R, Choti MA. Preoperative chemotherapy for colorectal liver metastases: impact on hepatic histology and postoperative outcome. J Gastrointest Surg. 2007; 11:860–868. PMID: 17492335.
Article19. Ward J, Guthrie JA, Sheridan MB, Boyes S, Smith JT, Wilson D, et al. Sinusoidal obstructive syndrome diagnosed with superparamagnetic iron oxide-enhanced magnetic resonance imaging in patients with chemotherapy-treated colorectal liver metastases. J Clin Oncol. 2008; 26:4304–4310. PMID: 18779617.
Article20. Rubbia-Brandt L, Lauwers GY, Wang H, Majno PE, Tanabe K, Zhu AX, et al. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology. 2010; 56:430–439. PMID: 20459550.
Article21. Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006; 24:2065–2072. PMID: 16648507.
Article22. Fernandez FG, Ritter J, Goodwin JW, Linehan DC, Hawkins WG, Strasberg SM. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005; 200:845–853. PMID: 15922194.
Article23. Kim GB, Kwon JH, Kang DS. Hypereosinophilic syndrome: imaging findings in patients with hepatic involvement. AJR Am J Roentgenol. 1993; 161:577–580. PMID: 8352109.
Article24. Yoon IL. The eosinophil and gastrointestinal carcinoma. Am J Surg. 1959; 97:195–200. PMID: 13617573.
Article25. Iwasaki K, Torisu M, Fujimura T. Malignant tumor and eosinophils. I. Prognostic significance in gastric cancer. Cancer. 1986; 58:1321–1327. PMID: 3742457.
Article26. Hong SW, Kim HG, Park CI, Lee SI. Eosinophilic liver abscess in patients with gastric carcinoma. Korean J Pathol. 1993; 27:27–33.27. Won JH, Kim MJ, Kim BM, Ji H, Chung JJ, Yoo HS, et al. Focal eosinophilic infiltration of the liver: a mimick of hepatic metastasis. Abdom Imaging. 1999; 24:369–372. PMID: 10390559.
Article28. Han NY, Park BJ, Kim MJ, Sung DJ, Cho SB. Hepatic parenchymal heterogeneity on contrast-enhanced CT scans following oxaliplatin-based chemotherapy: natural history and association with clinical evidence of sinusoidal obstruction syndrome. Radiology. 2015; 276:766–774. PMID: 25822471.
Article29. Yoo SY, Han JK, Kim YH, Kim TK, Choi BI, Han MC. Focal eosinophilic infiltration in the liver: radiologic findings and clinical course. Abdom Imaging. 2003; 28:326–332. PMID: 12719902.
Article30. Hong SW, Cho MY, Park C. Expression of eosinophil chemotactic factors in stomach cancer. Yonsei Med J. 1999; 40:131–136. PMID: 10333716.
Article31. Bateman JR, Pugh RP, Cassidy FR, Marshall GJ, Irwin LE. 5-fluorouracil given once weekly: comparison of intravenous and oral administration. Cancer. 1971; 28:907–913. PMID: 5111743.
Article32. Aloia T, Sebagh M, Plasse M, Karam V, Lévi F, Giacchetti S, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006; 24:4983–4990. PMID: 17075116.
Article33. Twelves C, Glynne-Jones R, Cassidy J, Schüller J, Goggin T, Roos B, et al. Effect of hepatic dysfunction due to liver metastases on the pharmacokinetics of capecitabine and its metabolites. Clin Cancer Res. 1999; 5:1696–1702. PMID: 10430071.34. Willmot FC, Robertson GW. Senecio disease, or cirrhosis of the liver due to senecio poisoning. Lancet. 1920; 196:848–849.
Article35. DeLeve LD, McCuskey RS, Wang X, Hu L, McCuskey MK, Epstein RB, et al. Characterization of a reproducible rat model of hepatic veno-occlusive disease. Hepatology. 1999; 29:1779–1791. PMID: 10347121.
Article36. Nakano H, Oussoultzoglou E, Rosso E, Casnedi S, Chenard-Neu MP, Dufour P, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008; 247:118–124. PMID: 18156931.
Article37. Mehta NN, Ravikumar R, Coldham CA, Buckels JA, Hubscher SG, Bramhall SR, et al. Effect of preoperative chemotherapy on liver resection for colorectal liver metastases. Eur J Surg Oncol. 2008; 34:782–786. PMID: 18160247.
Article38. Julie C, Lutz MP, Aust DA, Kandutsch S, Collette L, Praet M. Pathological analysis of hepatic injury after oxaliplatin-based neoadjuvant chemotherapy of colorectal cancer liver metastases: results of the EORTC Intergroup phase III study 40983 [abstract 241]. In : ASCO 2007 Gastrointestinal Cancers Symposium; Orlando. 2007.39. DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis. 2002; 22:27–42. PMID: 11928077.
Article40. Robinson PJ. The effects of cancer chemotherapy on liver imaging. Eur Radiol. 2009; 19:1752–1762. PMID: 19238392.
Article41. Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007; 94:274–286. PMID: 17315288.
Article42. Deleve LD, Wang X, Tsai J, Kanel G, Strasberg S, Tokes ZA. Sinusoidal obstruction syndrome (veno-occlusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology. 2003; 125:882–890. PMID: 12949732.43. Lassau N, Leclère J, Auperin A, Bourhis JH, Hartmann O, Valteau-Couanet D, et al. Hepatic veno-occlusive disease after myeloablative treatment and bone marrow transplantation: value of gray-scale and Doppler US in 100 patients. Radiology. 1997; 204:545–552. PMID: 9240551.
Article44. Erturk SM, Mortelé KJ, Binkert CA, Glickman JN, Oliva MR, Ros PR, et al. CT features of hepatic venoocclusive disease and hepatic graft-versus-host disease in patients after hematopoietic stem cell transplantation. AJR Am J Roentgenol. 2006; 186:1497–1501. PMID: 16714636.
Article45. Shin NY, Kim MJ, Lim JS, Park MS, Chung YE, Choi JY, et al. Accuracy of gadoxetic acid-enhanced magnetic resonance imaging for the diagnosis of sinusoidal obstruction syndrome in patients with chemotherapy-treated colorectal liver metastases. Eur Radiol. 2012; 22:864–871. PMID: 22108766.
Article46. Uchino K, Fujisawa M, Watanabe T, Endo Y, Nobuhisa T, Matsumoto Y, et al. Oxaliplatin-induced liver injury mimicking metastatic tumor on images: a case report. Jpn J Clin Oncol. 2013; 43:1034–1038. PMID: 23958518.
Article47. Choi JH, Won YW, Kim HS, Oh YH, Lim S, Kim HJ. Oxaliplatin-induced sinusoidal obstruction syndrome mimicking metastatic colon cancer in the liver. Oncol Lett. 2016; 11:2861–2864. PMID: 27073565.
Article48. Khan AZ, Morris-Stiff G, Makuuchi M. Patterns of chemotherapy-induced hepatic injury and their implications for patients undergoing liver resection for colorectal liver metastases. J Hepatobiliary Pancreat Surg. 2009; 16:137–144. PMID: 19093069.
Article49. McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006; 40(Suppl 1):S17–S29. PMID: 16540762.50. Morris-Stiff G, Tan YM, Vauthey JN. Hepatic complications following preoperative chemotherapy with oxaliplatin or irinotecan for hepatic colorectal metastases. Eur J Surg Oncol. 2008; 34:609–614. PMID: 17764887.
Article51. Oliva MR, Mortele KJ, Segatto E, Glickman JN, Erturk SM, Ros PR, et al. Computed tomography features of nonalcoholic steatohepatitis with histopathologic correlation. J Comput Assist Tomogr. 2006; 30:37–43. PMID: 16365570.
Article52. Cho ES, Yu JS, Kim MJ, Kim JH, Chung JJ, Kim KW. Focal eosinophilic necrosis on superparamagnetic iron oxide-enhanced MRI. AJR Am J Roentgenol. 2010; 194:1296–1302. PMID: 20410417.
Article53. Lowe D, Jorizzo J, Hutt MS. Tumour-associated eosinophilia: a review. J Clin Pathol. 1981; 34:1343–1348. PMID: 7035499.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Primary hepatic tuberculosis: A rare occurrence
- Focal Hepatic Lesions with Peripheral Eosinophilia: Imaging Features of Various Disease
- Current Status and Recent Update of Imaging Evaluation for Peri-Hilar Cholangiocarcinoma
- Ultrasonic diagnosis of hepatic metastases in patients with stomach cancer and colon cancer
- F-18 FDG Uptake in an Eosinophilic Liver Abscess Mimicking Hepatic Metastasis on PET/CT Images