Yonsei Med J.
2016 Nov;57(6):1354-1360. 10.3349/ymj.2016.57.6.1354.
Immunogenicity and Safety of Trivalent Split Influenza Vaccine in Healthy Korean Adults with Low Pre-Existing Antibody Levels: An Open Phase I Trial
- Affiliations
-
- 1The Vaccine Bio Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 2Department of Clinical Pharmacology and Therapeutics, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. yimds@catholic.ac.kr
- 3Department of Pediatrics, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2427152
- DOI: http://doi.org/10.3349/ymj.2016.57.6.1354
Abstract
- PURPOSE
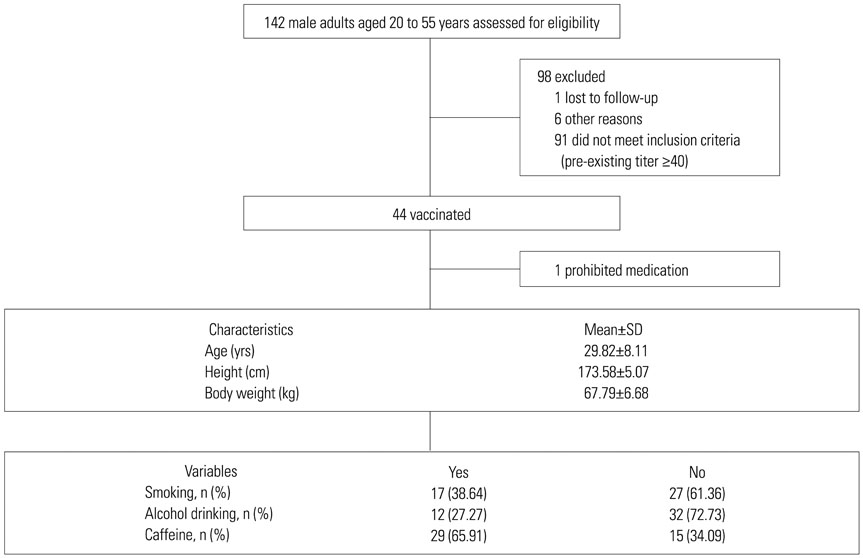
A phase I clinical trial was conducted to evaluate the immunogenicity and safety of newly developed egg-cultivated trivalent inactivated split influenza vaccine (TIV) in Korea.
MATERIALS AND METHODS
The TIV was administered to 43 healthy male adults. Subjects with high pre-existing titers were excluded in a screening step. Immune response was measured by a hemagglutination inhibition (HI) assay.
RESULTS
The seroprotection rates against A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2) and B/Brisbane/60/2009 were 74.42% [95% confidence interval (CI): 61.38-87.46], 72.09% (95% CI: 58.69-85.50), and 86.05% (95% CI: 75.69-96.40), respectively. Calculated seroconversion rates were 74.42% (95% CI: 61.38-87.46), 74.42% (95% CI: 61.38-87.46), and 79.07% (95% CI: 66.91-91.23), respectively. There were 25 episodes of solicited local adverse events in 21 subjects (47.73%), 21 episodes of solicited general adverse events in 16 subjects (36.36%) and 5 episodes of unsolicited adverse events in 5 subjects (11.36%). All adverse events were grade 1 or 2 and disappeared within three days.
CONCLUSION
The immunogenicity and safety of TIV established in this phase I trial are sufficient to plan a larger scale clinical trial.
Keyword
MeSH Terms
-
Adult
Antibodies, Viral/*blood
Asian Continental Ancestry Group
Female
Hemagglutination Inhibition Tests
Humans
Influenza A Virus, H1N1 Subtype/*immunology
Influenza A Virus, H3N2 Subtype/*immunology
Influenza Vaccines/administration & dosage/*adverse effects/immunology
Influenza, Human/blood/immunology/prevention & control
Male
Middle Aged
Republic of Korea
Vaccination/*methods
Vaccines, Inactivated/administration & dosage/adverse effects/immunology
Antibodies, Viral
Influenza Vaccines
Vaccines, Inactivated
Figure
Reference
-
1. World Health Organization. Influenza. accessed on 2016 Mar 1. Available at: http://www.who.int/mediacentre/factsheets/fs211/en.2. Fiore AE, Shay DK, Haber P, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep. 2007; 56:1–54.3. Sullivan KM. Health impact of influenza in the United States. Pharmacoeconomics. 1996; 9:Suppl 3. 26–33.
Article4. Centers for Disease Control and Prevention (CDC). Key facts about influenza (flu) & flu vaccine. accessed on 2016 Jan 10. Available at: http://www.cdc.gov/flu/keyfacts.htm.5. de Jong MD, Tran TT, Truong HK, Vo MH, Smith GJ, Nguyen VC, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005; 353:2667–2672.
Article6. Le QM, Kiso M, Someya K, Sakai YT, Nguyen TH, Nguyen KH, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005; 437:1108.7. Influenza vaccines. Wkly Epidemiol Rec. 2002; 77:230–239.8. Nichol KL. The efficacy, effectiveness and cost-effectiveness of inactivated influenza virus vaccines. Vaccine. 2003; 21:1769–1775.
Article9. Pyhälä R, Alanko S, Forsten T, Haapa K, Kinnunen L, Jääskivi M, et al. Early kinetics of antibody response to inactivated influenza vaccine. Clin Diagn Virol. 1994; 1:271–278.
Article10. Gulati U, Kumari K, Wu W, Keitel WA, Air GM. Amount and avidity of serum antibodies against native glycoproteins and denatured virus after repeated influenza whole-virus vaccination. Vaccine. 2005; 23:1414–1425.
Article11. Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 2004; 103:133–138.
Article12. Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull. 1979; 35:69–75.
Article13. Food and Drug Administration Center for Biologics Evaluation and Research. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. accessed on 2016 Apr 30. Available at: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074775.htm.14. Food and Drug Administration Center for Biologics Evaluation and Research. Guidance for industry: clinical data needed to support the licensure of seasonal inactivated influenza vaccines. accessed on 2016 Apr 30. Available at: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074794.htm.15. Feng SZ, Jiao PR, Qi WB, Fan HY, Liao M. Development and strategies of cell-culture technology for influenza vaccine. Appl Microbiol Biotechnol. 2011; 89:893–902.
Article16. Minor PD, Engelhardt OG, Wood JM, Robertson JS, Blayer S, Colegate T, et al. Current challenges in implementing cell-derived influenza vaccines: implications for production and regulation, July 2007, NIBSC, Potters Bar, UK. Vaccine. 2009; 27:2907–2913.
Article17. Schild GC, Oxford JS, de Jong JC, Webster RG. Evidence for host-cell selection of influenza virus antigenic variants. Nature. 1983; 303:706–709.
Article18. Oxford JS, Corcoran T, Knott R, Bates J, Bartolomei O, Major D, et al. Serological studies with influenza A(H1N1) viruses cultivated in eggs or in a canine kidney cell line (MDCK). Bull World Health Organ. 1987; 65:181–187.19. Katz JM, Webster RG. Amino acid sequence identity between the HA1 of influenza A (H3N2) viruses grown in mammalian and primary chick kidney cells. J Gen Virol. 1992; 73(Pt 5):1159–1165.
Article20. Szymczakiewicz-Multanowska A, Groth N, Bugarini R, Lattanzi M, Casula D, Hilbert A, et al. Safety and immunogenicity of a novel influenza subunit vaccine produced in mammalian cell culture. J Infect Dis. 2009; 200:841–848.
Article21. Reisinger KS, Block SL, Izu A, Groth N, Holmes SJ. Subunit influenza vaccines produced from cell culture or in embryonated chicken eggs: comparison of safety, reactogenicity, and immunogenicity. J Infect Dis. 2009; 200:849–857.
Article22. Groth N, Montomoli E, Gentile C, Manini I, Bugarini R, Podda A. Safety, tolerability and immunogenicity of a mammalian cell-culture-derived influenza vaccine: a sequential Phase I and Phase II clinical trial. Vaccine. 2009; 27:786–791.
Article23. Nakamura K, Homma M. Protein synthesis in Vero cells abortively infected with influenza B virus. J Gen Virol. 1981; 56(Pt 1):199–202.
Article24. Lau SC, Scholtissek C. Abortive infection of Vero cells by an influenza A virus (FPV). Virology. 1995; 212:225–231.
Article25. Robertson JS, Cook P, Attwell AM, Williams SP. Replicative advantage in tissue culture of egg-adapted influenza virus over tissue-culture derived virus: implications for vaccine manufacture. Vaccine. 1995; 13:1583–1588.
Article26. Roedig JV, Rapp E, Höper D, Genzel Y, Reichl U. Impact of host cell line adaptation on quasispecies composition and glycosylation of influenza A virus hemagglutinin. PLoS One. 2011; 6:e27989.
Article27. Lin YP, Wharton SA, Martín J, Skehel JJ, Wiley DC, Steinhauer DA. Adaptation of egg-grown and transfectant influenza viruses for growth in mammalian cells: selection of hemagglutinin mutants with elevated pH of membrane fusion. Virology. 1997; 233:402–410.
Article28. Hess RD, Weber F, Watson K, Schmitt S. Regulatory, biosafety and safety challenges for novel cells as substrates for human vaccines. Vaccine. 2012; 30:2715–2727.
Article29. Perdue ML, Arnold F, Li S, Donabedian A, Cioce V, Warf T, et al. The future of cell culture-based influenza vaccine production. Expert Rev Vaccines. 2011; 10:1183–1194.
Article30. Schultz-Cherry S, Jones JC. Influenza vaccines: the good, the bad, and the eggs. Adv Virus Res. 2010; 77:63–84.31. Baxter R, Patriarca PA, Ensor K, Izikson R, Goldenthal KL, Cox MM. Evaluation of the safety, reactogenicity and immunogenicity of FluBlok® trivalent recombinant baculovirus-expressed hemagglutinin influenza vaccine administered intramuscularly to healthy adults 50-64 years of age. Vaccine. 2011; 29:2272–2278.
Article32. López-Macías C, Ferat-Osorio E, Tenorio-Calvo A, Isibasi A, Talavera J, Arteaga-Ruiz O, et al. Safety and immunogenicity of a virus-like particle pandemic influenza A (H1N1) 2009 vaccine in a blinded, randomized, placebo-controlled trial of adults in Mexico. Vaccine. 2011; 29:7826–7834.
Article33. Rappuoli R, Giudice GD. Influenza vaccines for the future. 2nd ed. Basel: Springer;2011.34. Van Kampen KR, Shi Z, Gao P, Zhang J, Foster KW, Chen DT, et al. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine. 2005; 23:1029–1036.
Article35. Hegde NR. Cell culture-based influenza vaccines: a necessary and indispensable investment for the future. Hum Vaccin Immunother. 2015; 11:1223–1234.
Article36. Bandell AR, Simões EA. Live attenuated influenza vaccine tetravalent: a clinical review. Expert Rev Vaccines. 2015; 14:963–973.
Article37. Valero-Pacheco N, Pérez-Toledo M, Villasís-Keever MÁ, Núñez-Valencia A, Boscó-Gárate I, Lozano-Dubernard B, et al. Antibody persistence in adults two years after vaccination with an H1N1 2009 pandemic influenza virus-like particle vaccine. PLoS One. 2016; 11:e0150146.
Article38. Dormitzer PR, Tsai TF, Del Giudice G. New technologies for influenza vaccines. Hum Vaccin Immunother. 2012; 8:45–58.
Article39. Hirota Y, Kaji M, Ide S, Goto S, Oka T. The hemagglutination inhibition antibody responses to an inactivated influenza vaccine among healthy adults: with special reference to the prevaccination antibody and its interaction with age. Vaccine. 1996; 14:1597–1602.
Article40. Beyer WE, Palache AM, Sprenger MJ, Hendriksen E, Tukker JJ, Darioli R, et al. Effects of repeated annual influenza vaccination on vaccine sero-response in young and elderly adults. Vaccine. 1996; 14:1331–1339.
Article41. Nabeshima S, Kashiwagi K, Murata M, Kanamoto Y, Furusyo N, Hayashi J. Antibody response to influenza vaccine in adults vaccinated with identical vaccine strains in consecutive years. J Med Virol. 2007; 79:320–325.
Article42. Gross PA, Sperber SJ, Donabedian A, Dran S, Morchel G, Cataruozolo P, et al. Paradoxical response to a novel influenza virus vaccine strain: the effect of prior immunization. Vaccine. 1999; 17:2284–2289.
Article43. Iorio AM, Camilloni B, Basileo M, Neri M, Lepri E, Spighi M. Effects of repeated annual influenza vaccination on antibody responses against unchanged vaccine antigens in elderly frail institutionalized volunteers. Gerontology. 2007; 53:411–418.
Article44. Kelly HA, Skowronski DM, De Serres G, Effler PV. Adverse events associated with 2010 CSL and other inactivated influenza vaccines. Med J Aust. 2011; 195:318–320.
Article45. Skowronski DM, Strauss B, De Serres G, MacDonald D, Marion SA, Naus M, et al. Oculo-respiratory syndrome: a new influenza vaccine-associated adverse event? Clin Infect Dis. 2003; 36:705–713.
Article46. Nicholson KG, Webster RG, Hay AJ. Textbook of influenza. Oxford: Blackwell Science;1998.47. Therapeutic Goods Administration. Seasonal flu vaccine: overview of vaccine regulation and safety monitoring and investigation into adverse events following 2010 seasonal influenza vaccination in young children. accessed on 2016 Jan 10. Available at: https://www.tga.gov.au/node/581.48. Beyer WE, Nauta JJ, Palache AM, Giezeman KM, Osterhaus AD. Immunogenicity and safety of inactivated influenza vaccines in primed populations: a systematic literature review and meta-analysis. Vaccine. 2011; 29:5785–5792.
Article49. Song JY, Cheong HJ, Woo HJ, Wie SH, Lee JS, Chung MH, et al. Immunogenicity and safety of trivalent inactivated influenza vaccine: a randomized, double-blind, multi-center, phase 3 clinical trial in a vaccine-limited country. J Korean Med Sci. 2011; 26:191–195.
Article50. Treanor JJ, Campbell JD, Brady RC, Keitel WA, Drame M, Jain VK, et al. Rapid licensure of a new, inactivated influenza vaccine in the United States. Hum Vaccin. 2005; 1:239–244.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunogenicity and Safety of Trivalent Inactivated Influenza Vaccine: A Randomized, Double-Blind, Multi-Center, Phase 3 Clinical Trial in a Vaccine-Limited Country
- Safety and Immunogenicity of an Egg-Cultivated Quadrivalent Inactivated Split-virion Influenza Vaccine (GC3110A) in Healthy Korean Children: a Randomized, Double-blinded, Active-controlled Phase III Study
- Immunogenicity and Safety of Inactivated Influenza Vaccine in Healthy Korean Children and Adolescent
- Safety and Immunogenicity of a New Trivalent Inactivated Split-virus Influenza Vaccine in Healthy Korean Children: A Randomized, Double-blinded, Active-controlled, Phase III Study
- Comparison of Split versus Subunit Seasonal Influenza Vaccine in Korean Children over 3 to under 18 Years of Age