Yonsei Med J.
2016 Nov;57(6):1329-1338. 10.3349/ymj.2016.57.6.1329.
Arginase Inhibition Restores Peroxynitrite-Induced Endothelial Dysfunction via L-Arginine-Dependent Endothelial Nitric Oxide Synthase Phosphorylation
- Affiliations
-
- 1Department of Biology, College of Natural Sciences, School of Medicine, Kangwon National University, Chuncheon, Korea. ryoosw08@kangwon.ac.kr
- 2Department of Anesthesiology and Pain Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea. hyunkyolim@yonsei.ac.kr
- 3Infectious Signaling Network Research Center, Department of Physiology, School of Medicine, Chungnam National University, Daejeon, Korea.
- 4Department of New Drug Discovery and Development, Chungnam National University, Daejeon, Korea.
- 5Department of Molecular and Cellular Biochemistry, School of Medicine, Kangwon National University, Chuncheon, Korea.
- KMID: 2427149
- DOI: http://doi.org/10.3349/ymj.2016.57.6.1329
Abstract
- PURPOSE
Peroxynitrite plays a critical role in vascular pathophysiology by increasing arginase activity and decreasing endothelial nitric oxide synthase (eNOS) activity. Therefore, the aims of this study were to investigate whether arginase inhibition and L-arginine supplement could restore peroxynitrite-induced endothelial dysfunction and determine the involved mechanism.
MATERIALS AND METHODS
Human umbilical vein endothelial cells (HUVECs) were treated with SIN-1, a peroxynitrite generator, and arginase activity, nitrite/nitrate production, and expression levels of proteins were measured. eNOS activation was evaluated via Western blot and dimer blot analysis. We also tested nitric oxide (NO) and reactive oxygen species (ROS) production and performed a vascular tension assay.
RESULTS
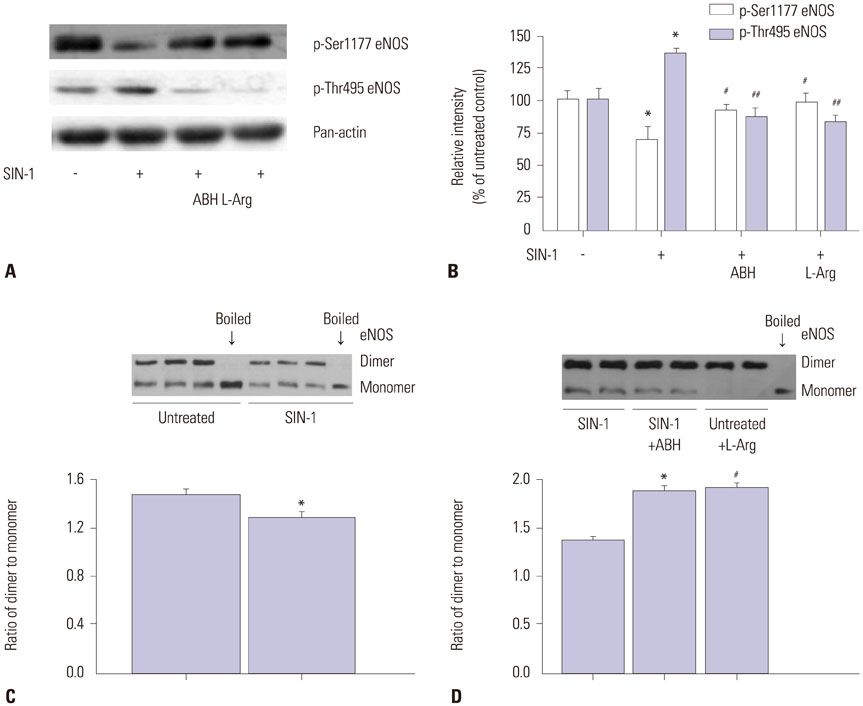
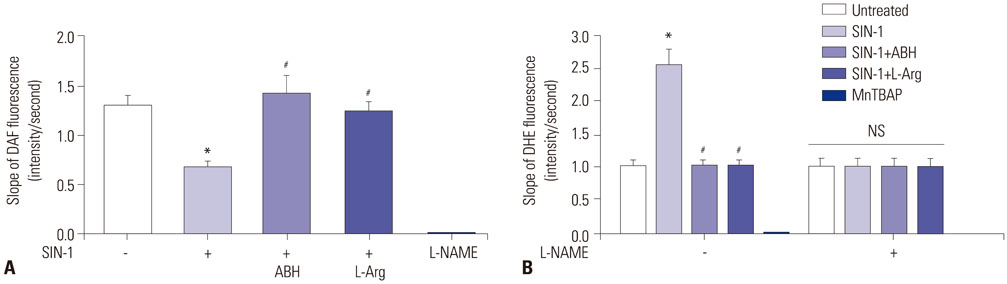
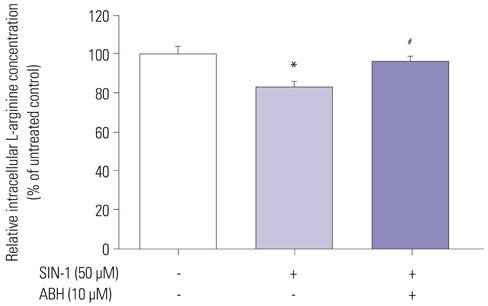
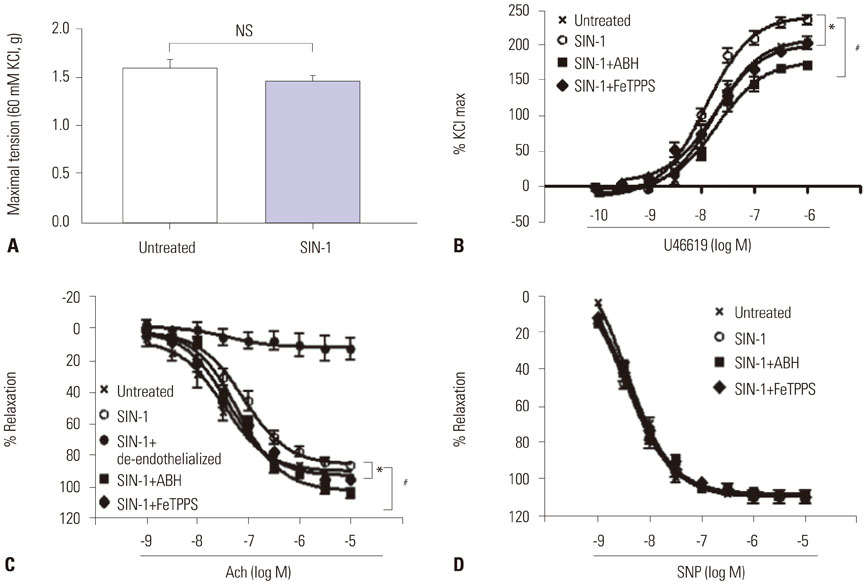
SIN-1 treatment increased arginase activity in a time- and dose-dependent manner and reciprocally decreased nitrite/nitrate production that was prevented by peroxynitrite scavenger in HUVECs. Furthermore, SIN-1 induced an increase in the expression level of arginase I and II, though not in eNOS protein. The decreased eNOS phosphorylation at Ser1177 and the increased at Thr495 by SIN-1 were restored with arginase inhibitor and L-arginine. The changed eNOS phosphorylation was consistent in the stability of eNOS dimers. SIN-1 decreased NO production and increased ROS generation in the aortic endothelium, all of which was reversed by arginase inhibitor or L-arginine. N(G)-Nitro-L-arginine methyl ester (L-NAME) prevented SIN-1-induced ROS generation. In the vascular tension assay, SIN-1 enhanced vasoconstrictor responses to U46619 and attenuated vasorelaxant responses to acetylcholine that were reversed by arginase inhibition.
CONCLUSION
These findings may explain the beneficial effect of arginase inhibition and L-arginine supplement on endothelial dysfunction under redox imbalance-dependent pathophysiological conditions.
Keyword
MeSH Terms
-
Animals
Arginase/*antagonists & inhibitors/metabolism
Arginine/analogs & derivatives/*metabolism
Endothelium, Vascular
Human Umbilical Vein Endothelial Cells/*drug effects/metabolism
Humans
Nitric Oxide/*metabolism
Nitric Oxide Synthase Type III/drug effects/*metabolism
Peroxynitrous Acid
Phosphorylation/*drug effects
Reactive Oxygen Species/metabolism
Vascular Diseases
Reactive Oxygen Species
Peroxynitrous Acid
Nitric Oxide
Arginine
Nitric Oxide Synthase Type III
Arginase
Figure
Cited by 1 articles
-
Arginase Inhibition Suppresses Native Low-Density Lipoprotein-Stimulated Vascular Smooth Muscle Cell Proliferation by NADPH Oxidase Inactivation
Bon-Hyeock Koo, Bong-Gu Yi, Wi-Kwang Wang, In-Young Ko, Kwang-Lae Hoe, Young-Guen Kwon, Moo-Ho Won, Young-Myeong Kim, Hyun Kyo Lim, Sungwoo Ryoo
Yonsei Med J. 2018;59(3):366-375. doi: 10.3349/ymj.2018.59.3.366.
Reference
-
1. Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003; 108:2000–2006.
Article2. Demougeot C, Prigent-Tessier A, Marie C, Berthelot A. Arginase inhibition reduces endothelial dysfunction and blood pressure rising in spontaneously hypertensive rats. J Hypertens. 2005; 23:971–978.
Article3. Ryoo S, Lemmon CA, Soucy KG, Gupta G, White AR, Nyhan D, et al. Oxidized low-density lipoprotein-dependent endothelial arginase II activation contributes to impaired nitric oxide signaling. Circ Res. 2006; 99:951–960.
Article4. White AR, Ryoo S, Li D, Champion HC, Steppan J, Wang D, et al. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension. 2006; 47:245–251.
Article5. Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, et al. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res. 2008; 102:923–932.6. Santhanam L, Lim HK, Lim HK, Miriel V, Brown T, Patel M, et al. Inducible NO synthase dependent S-nitrosylation and activation of arginase1 contribute to age-related endothelial dysfunction. Circ Res. 2007; 101:692–702.
Article7. Hwang HM, Lee JH, Min BS, Jeon BH, Hoe KL, Kim YM, et al. A novel arginase inhibitor derived from scutellavia indica restored endothelial function in ApoE-null mice fed a high-cholesterol diet. J Pharmacol Exp Ther. 2015; 355:57–65.
Article8. Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006; 113:1708–1714.9. Belik J, Stevens D, Pan J, McIntyre BA, Kantores C, Ivanovska J, et al. Pulmonary vascular and cardiac effects of peroxynitrite decomposition in newborn rats. Free Radic Biol Med. 2010; 49:1306–1314.
Article10. Sankaralingam S, Xu H, Davidge ST. Arginase contributes to endothelial cell oxidative stress in response to plasma from women with preeclampsia. Cardiovasc Res. 2010; 85:194–203.
Article11. Böger RH, Bode-Böger SM, Mügge A, Kienke S, Brandes R, Dwenger A, et al. Supplementation of hypercholesterolaemic rabbits with L-arginine reduces the vascular release of superoxide anions and restores NO production. Atherosclerosis. 1995; 117:273–284.
Article12. Mohr S, Stamler JS, Brüne B. Mechanism of covalent modification of glyceraldehyde-3-phosphate dehydrogenase at its active site thiol by nitric oxide, peroxynitrite and related nitrosating agents. FEBS Lett. 1994; 348:223–227.
Article13. Martin-Romero FJ, Gutiérrez-Martin Y, Henao F, Gutiérrez-Merino C. Fluorescence measurements of steady state peroxynitrite production upon SIN-1 decomposition: NADH versus dihydrodichlorofluorescein and dihydrorhodamine 123. J Fluoresc. 2004; 14:17–23.
Article14. Blum A, Hathaway L, Mincemoyer R, Schenke WH, Kirby M, Csako G, et al. Oral L-arginine in patients with coronary artery disease on medical management. Circulation. 2000; 101:2160–2164.
Article15. Dudek D, Legutko J, Heba G, Bartus S, Partyka L, Huk I, et al. L-arginine supplementation does not inhibit neointimal formation after coronary stenting in human beings: an intravascular ultrasound study. Am Heart J. 2004; 147:E12.
Article16. Walker HA, McGing E, Fisher I, Böger RH, Bode-Böger SM, Jackson G, et al. Endothelium-dependent vasodilation is independent of the plasma L-arginine/ADMA ratio in men with stable angina: lack of effect of oral L-arginine on endothelial function, oxidative stress and exercise performance. J Am Coll Cardiol. 2001; 38:499–505.
Article17. Wilson AM, Harada R, Nair N, Balasubramanian N, Cooke JP. L-arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation. 2007; 116:188–195.
Article18. Böger RH. L-Arginine therapy in cardiovascular pathologies: beneficial or dangerous? Curr Opin Clin Nutr Metab Care. 2008; 11:55–61.
Article19. Ansel GM, Lumsden AB. Evolving modalities for femoropopliteal interventions. J Endovasc Ther. 2009; 16:2 Suppl 2. II82–II97.
Article20. Griffiths MJ, Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med. 2005; 353:2683–2695.
Article21. Ichinose F, Roberts JD Jr, Zapol WM. Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation. 2004; 109:3106–3111.22. Barbato JE, Kibbe MR, Tzeng E. The emerging role of gene therapy in the treatment of cardiovascular diseases. Crit Rev Clin Lab Sci. 2003; 40:499–545.
Article23. Kibbe MR, Tzeng E. Nitric oxide synthase gene therapy in vascular pathology. Semin Perinatol. 2000; 24:51–54.
Article24. Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, et al. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008; 102:95–102.
Article25. Matsumoto A, Momomura S, Hirata Y, Aoyagi T, Sugiura S, Omata M. Inhaled nitric oxide and exercise capacity in congestive heart failure. Lancet. 1997; 349:999–1000.
Article26. Roger N, Barberà JA, Roca J, Rovira I, Gómez FP, Rodriguez-Roisin R. Nitric oxide inhalation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997; 156(3 Pt 1):800–806.
Article27. Steppan J, Ryoo S, Schuleri KH, Gregg C, Hasan RK, White AR, et al. Arginase modulates myocardial contractility by a nitric oxide synthase 1-dependent mechanism. Proc Natl Acad Sci U S A. 2006; 103:4759–4764.
Article28. Morris SM Jr. Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol. 2009; 157:922–930.
Article29. Shatanawi A, Romero MJ, Iddings JA, Chandra S, Umapathy NS, Verin AD, et al. Angiotensin II-induced vascular endothelial dysfunction through RhoA/Rho kinase/p38 mitogen-activated protein kinase/arginase pathway. Am J Physiol Cell Physiol. 2011; 300:C1181–C1192.
Article30. Dunn J, Gutbrod S, Webb A, Pak A, Jandu SK, Bhunia A, et al. S-nitrosation of arginase 1 requires direct interaction with inducible nitric oxide synthase. Mol Cell Biochem. 2011; 355:83–89.
Article31. Ming XF, Barandier C, Viswambharan H, Kwak BR, Mach F, Mazzolai L, et al. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction. Circulation. 2004; 110:3708–3714.
Article32. Heo J, Campbell SL. Mechanism of redox-mediated guanine nucleotide exchange on redox-active Rho GTPases. J Biol Chem. 2005; 280:31003–31010.
Article33. Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996; 271(5 Pt 1):C1424–C1437.
Article34. Wang P, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. Evidence for peroxynitrite-mediated reperfusion injury. J Biol Chem. 1996; 271:29223–29230.
Article35. Torres-Dueñas D, Celes MR, Freitas A, Alves-Filho JC, Spiller F, Dal-Secco D, et al. Peroxynitrite mediates the failure of neutrophil migration in severe polymicrobial sepsis in mice. Br J Pharmacol. 2007; 152:341–352.
Article36. Salvemini D, Doyle TM, Cuzzocrea S. Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem Soc Trans. 2006; 34(Pt 5):965–970.
Article37. Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002; 109:817–826.
Article38. Chen W, Druhan LJ, Chen CA, Hemann C, Chen YR, Berka V, et al. Peroxynitrite induces destruction of the tetrahydrobiopterin and heme in endothelial nitric oxide synthase: transition from reversible to irreversible enzyme inhibition. Biochemistry. 2010; 49:3129–3137.
Article39. Venardos K, Zhang WZ, Lang C, Kaye DM. Effect of peroxynitrite on endothelial L-arginine transport and metabolism. Int J Biochem Cell Biol. 2009; 41:2522–2527.
Article40. Pritchard KA Jr, Groszek L, Smalley DM, Sessa WC, Wu M, Villalon P, et al. Native low-density lipoprotein increases endothelial cell nitric oxide synthase generation of superoxide anion. Circ Res. 1995; 77:510–518.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intravenous administration of piceatannol, an arginase inhibitor, improves endothelial dysfunction in aged mice
- Endothelial arginase II and atherosclerosis
- Korean Red Ginseng Water Extract Restores Impaired Endothelial Function by Inhibiting Arginase Activity in Aged Mice
- Effects of Regular Exercise on the Endothelial Function in Cardiovascular Disease
- Arginase Inhibition by Ethylacetate Extract of Caesalpinia sappan Lignum Contributes to Activation of Endothelial Nitric Oxide Synthase