Yonsei Med J.
2016 Nov;57(6):1312-1323. 10.3349/ymj.2016.57.6.1312.
Knockdown of the M2 Isoform of Pyruvate Kinase (PKM2) with shRNA Enhances the Effect of Docetaxel in Human NSCLC Cell Lines In Vitro
- Affiliations
-
- 1Department of Oncology, Jinshan Hospital, Medical Center of Fudan University, Shanghai, China. qiaotk@163.com
- 2Department of Radiotherapy, Donghua Hospital of Sun Yat-sen University, Dongguan, China.
- KMID: 2427147
- DOI: http://doi.org/10.3349/ymj.2016.57.6.1312
Abstract
- PURPOSE
The aim of our study was to explore the relationships between the M2 isoform of pyruvate kinase (PKM2) and the sensitivity of human non-small cell lung cancer (NSCLC) cells to docetaxel in vitro.
MATERIALS AND METHODS
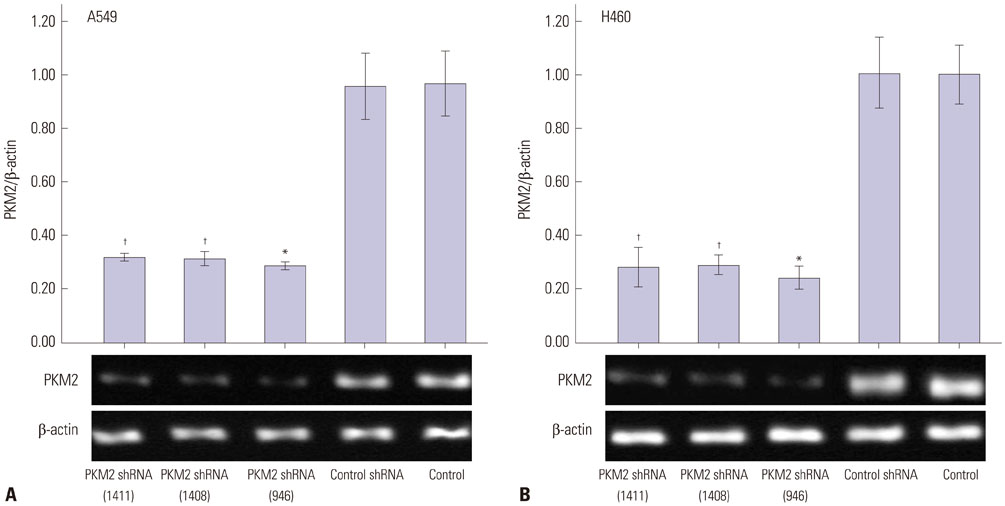
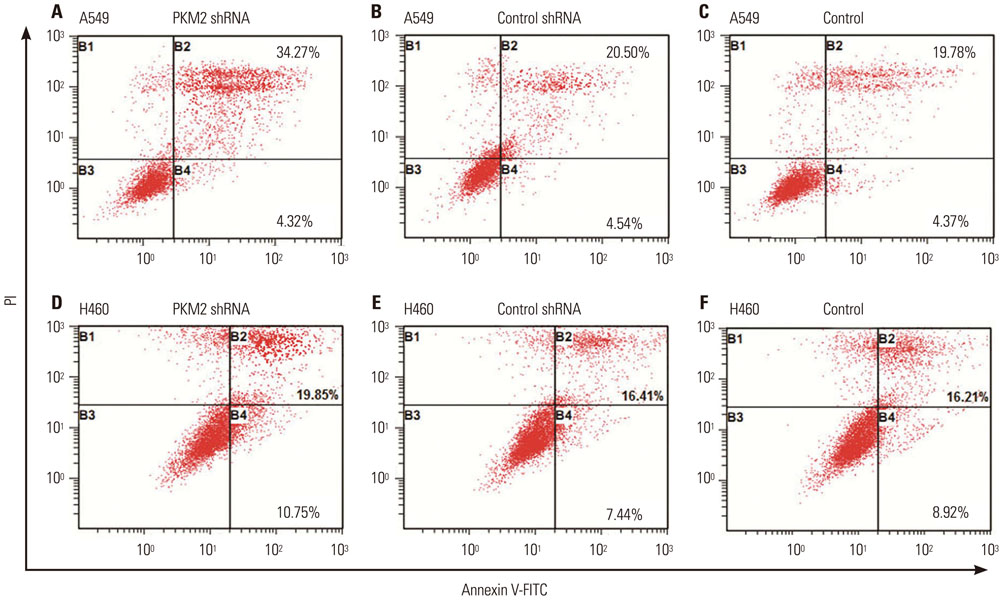
With the method of plasmid transfection, we silenced the expression of PKM2 successfully in A549 and H460 cells. Western blotting and real-time PCR were applied to detect PKM2 expression at protein and gene levels. Cell viability was examined by CCK8 assay. Cell cycle distribution and apoptosis were examined by flow cytometry. P21 and Bax were detected.
RESULTS
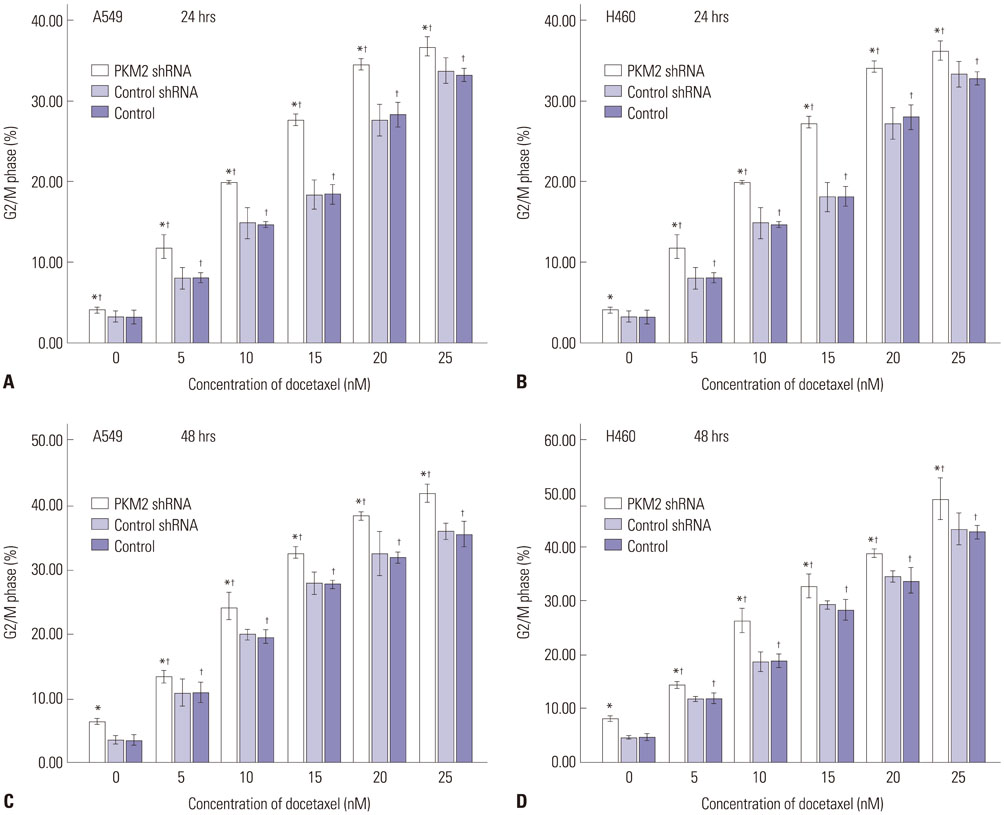
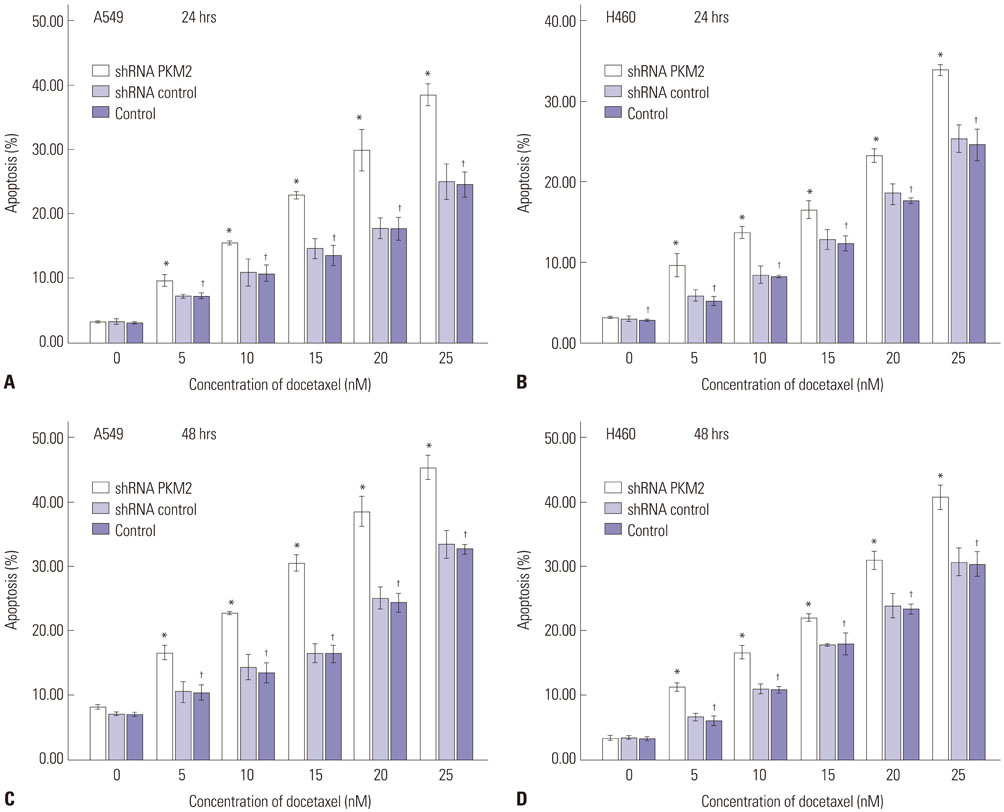
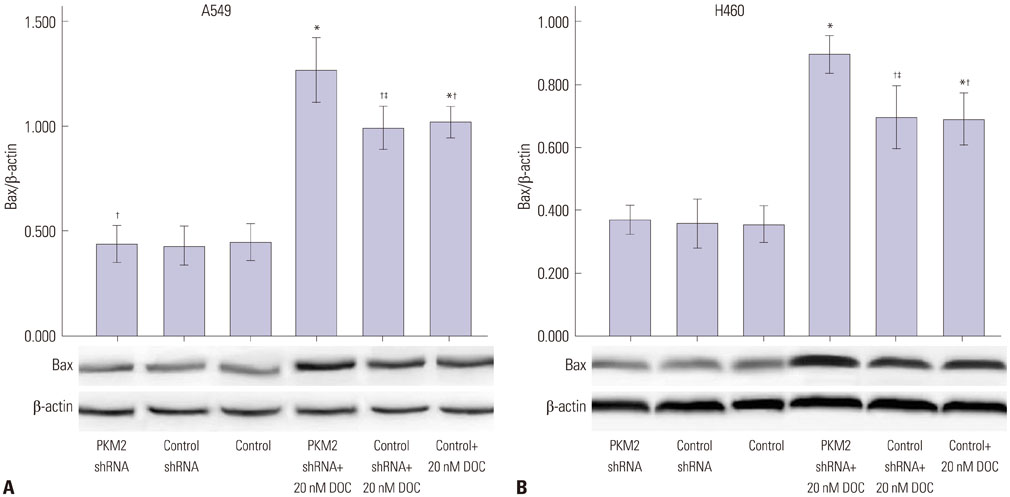
Expression of PKM2 mRNA and protein were significantly decreased by shRNA targeting PKM2. Silencing of PKM2 increased docetaxel sensitivity of human NSCLC A549 and H460 cells in a collaborative manner, resulting in strong suppression of cell viability. The results of flow cytometric assays suggested that knockdown of PKM2 or docetaxel treatment, whether used singly or in combination, blocked the cells in the G2/M phase, which is in consistent with the effect of the two on the expression of p21. Cells with PKM2 silencing were more likely to be induced into apoptosis by docetaxel although knockdown of PKM2 alone can't induce apoptosis significantly, which is in consistent with the effect of the two on Bax expression.
CONCLUSION
The results suggest that PKM2 knockdown could serve as a chemosensitizer to docetaxel in non-small lung cancer cells through targeting PKM2, leading to inhibition of cell viability, increase of cell arrest of G2/M phase and apoptosis.
Keyword
MeSH Terms
-
Apoptosis/*drug effects
Carcinoma, Non-Small-Cell Lung/drug therapy/genetics
Cell Cycle
Cell Line, Tumor
Cell Proliferation/*drug effects
Humans
Lung Neoplasms/genetics/*metabolism/pathology
MicroRNAs
Protein Isoforms
Pyruvate Kinase/*antagonists & inhibitors/genetics/metabolism
RNA, Small Interfering/genetics
Real-Time Polymerase Chain Reaction
Taxoids/*pharmacology
Transfection
Tumor Cells, Cultured
Up-Regulation/*drug effects
MicroRNAs
Protein Isoforms
RNA, Small Interfering
Taxoids
Pyruvate Kinase
Figure
Reference
-
1. Warburg O. On the origin of cancer cells. Science. 1956; 123:309–314.
Article2. Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol. 2011; 43:969–980.
Article3. Yeh CS, Wang JY, Chung FY, Lee SC, Huang MY, Kuo CW, et al. Significance of the glycolytic pathway and glycolysis related-genes in tumorigenesis of human colorectal cancers. Oncol Rep. 2008; 19:81–91.
Article4. Gumińska M, Ignacak J, Kedryna T, Stachurska MB. Tumor-specific pyruvate kinase isoenzyme M2 involved in biochemical strategy of energy generation in neoplastic cells. Acta Biochim Pol. 1997; 44:711–724.
Article5. Hacker HJ, Steinberg P, Bannasch P. Pyruvate kinase isoenzyme shift from L-type to M2-type is a late event in hepatocarcinogenesis induced in rats by a choline-deficient/DL-ethionine-supplemented diet. Carcinogenesis. 1998; 19:99–107.
Article6. Steinberg P, Klingelhöffer A, Schäfer A, Wüst G, Weisse G, Oesch F, et al. Expression of pyruvate kinase M2 in preneoplastic hepatic foci of N-nitrosomorpholine-treated rats. Virchows Arch. 1999; 434:213–220.
Article7. Mazurek S. Pyruvate kinase type M2: a key regulator within the tumour metabolome and a tool for metabolic profiling of tumours. Ernst Schering Found Symp Proc. 2007; 99–124.
Article8. Bluemlein K, Grüning NM, Feichtinger RG, Lehrach H, Kofler B, Ralser M. No evidence for a shift in pyruvate kinase PKM1 to PKM2 expression during tumorigenesis. Oncotarget. 2011; 2:393–400.
Article9. Liu Z, Feng JG, Tuersun A, Liu T, Liu H, Liu Q, et al. Proteomic identification of differentially-expressed proteins in esophageal cancer in three ethnic groups in Xinjiang. Mol Biol Rep. 2011; 38:3261–3269.
Article10. Yuan SJ, Qiao TK, Chen W, Zhuang XB. The expression of PKM2 in early stage human non-small cell lung cancer and its clinical significance. J Clin Intern Med. 2015; 32:524–527.11. Huang BT, Lu JY, Lin PX, Chen JZ, Kuang Y, Chen CZ. Comparison of Two RapidArc Delivery Strategies in Stereotactic Body Radiotherapy of Peripheral Lung Cancer with Flattening Filter Free Beams. PLoS One. 2015; 10:e0127501.
Article12. Chen Y, Han S, Zheng MJ, Xue Y, Liu WC. Clinical characteristics of 274 non-small cell lung cancer patients in China. Onkologie. 2013; 36:248–254.
Article13. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013; 63:11–30.
Article14. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002; 346:92–98.
Article15. Hsiao JR, Leu SF, Huang BM. Apoptotic mechanism of paclitaxel-induced cell death in human head and neck tumor cell lines. J Oral Pathol Med. 2009; 38:188–197.
Article16. Sun ML, Wang GJ, Li J, Cui JW, Zhang AL, Wang ZN, et al. [Construction of shRNA eukaryotic expression vectors of pkm2 gene and their effect on drug resistant cell line of acute promyelocytic leukemia]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010; 18:85–89.17. Yoo BC, Ku JL, Hong SH, Shin YK, Park SY, Kim HK, et al. Decreased pyruvate kinase M2 activity linked to cisplatin resistance in human gastric carcinoma cell lines. Int J Cancer. 2004; 108:532–539.
Article18. Li SL, Ye F, Cai WJ, Hu HD, Hu P, Ren H, et al. Quantitative proteome analysis of multidrug resistance in human ovarian cancer cell line. J Cell Biochem. 2010; 109:625–633.
Article19. Martinez-Balibrea E, Plasencia C, Ginés A, Martinez-Cardús A, Musulén E, Aguilera R, et al. A proteomic approach links decreased pyruvate kinase M2 expression to oxaliplatin resistance in patients with colorectal cancer and in human cell lines. Mol Cancer Ther. 2009; 8:771–778.
Article20. Shin YK, Yoo BC, Hong YS, Chang HJ, Jung KH, Jeong SY, et al. Upregulation of glycolytic enzymes in proteins secreted from human colon cancer cells with 5-fluorouracil resistance. Electrophoresis. 2009; 30:2182–2192.
Article21. Cortés-Cros M, Hemmerlin C, Ferretti S, Zhang J, Gounarides JS, Yin H, et al. M2 isoform of pyruvate kinase is dispensable for tumor maintenance and growth. Proc Natl Acad Sci U S A. 2013; 110:489–494.
Article22. Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008; 452:230–233.
Article23. Zha L, Qiao T, Yuan S, Lei L. Enhancement of radiosensitivity by CpG-oligodeoxyribonucleotide-7909 in human non-small cell lung cancer A549 cells. Cancer Biother Radiopharm. 2010; 25:165–170.
Article24. Zeng S, Chen YZ, Fu L, Johnson KR, Fan W. In vitro evaluation of schedule-dependent interactions between docetaxel and doxorubicin against human breast and ovarian cancer cells. Clin Cancer Res. 2000; 6:3766–3773.25. International Agency for Research on Cancer. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. accessed on. Available at: http://globocan.iarc.fr/Default.aspx.26. Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008; 452:181–186.
Article27. Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, et al. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell. 2012; 150:685–696.
Article28. Luo W, Semenza GL. Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol Metab. 2012; 23:560–566.
Article29. Gupta V, Bamezai RN. Human pyruvate kinase M2: a multifunctional protein. Protein Sci. 2010; 19:2031–2044.
Article30. Bissery MC, Guénard D, Guéritte-Voegelein F, Lavelle F. Experimental antitumor activity of taxotere (RP 56976, NSC 628503), a taxol analogue. Cancer Res. 1991; 51:4845–4852.31. Jurica MS, Mesecar A, Heath PJ, Shi W, Nowak T, Stoddard BL. The allosteric regulation of pyruvate kinase by fructose-1,6-bisphosphate. Structure. 1998; 6:195–210.
Article32. Martinez VG, O’Connor R, Liang Y, Clynes M. CYP1B1 expression is induced by docetaxel: effect on cell viability and drug resistance. Br J Cancer. 2008; 98:564–570.
Article33. Kucukzeybek Y, Gul MK, Cengiz E, Erten C, Karaca B, Gorumlu G, et al. Enhancement of docetaxel-induced cytotoxicity and apoptosis by all-trans retinoic acid (ATRA) through downregulation of survivin (BIRC5), MCL-1 and LTbeta-R in hormone- and drug resistant prostate cancer cell line, DU-145. J Exp Clin Cancer Res. 2008; 27:37.
Article34. Stark GR, Taylor WR. Control of the G2/M transition. Mol Biotechnol. 2006; 32:227–248.35. Torricelli C, Salvadori S, Valacchi G, Souček K, Slabáková E, Muscettola M, et al. Alternative pathways of cancer cell death by rottlerin: apoptosis versus autophagy. Evid Based Complement Alternat Med. 2012; 2012:980658.
Article36. Barboule N, Chadebech P, Baldin V, Vidal S, Valette A. Involvement of p21 in mitotic exit after paclitaxel treatment in MCF-7 breast adenocarcinoma cell line. Oncogene. 1997; 15:2867–2875.
Article37. Rossé T, Olivier R, Monney L, Rager M, Conus S, Fellay I, et al. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998; 391:496–499.
Article38. Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, et al. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003; 423:456–461.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects and Mechanisms of Metformin on the Proliferation of Esophageal Cancer Cells In Vitro and In Vivo
- Developmentally regulated GTP-binding protein 2 levels in prostate cancer cell lines impact docetaxel-induced apoptosis
- Targeting CD73 to Overcomes Resistance to First-Generation EGFR Tyrosine Kinase Inhibitors in Non–Small Cell Lung Cancer
- Phase II Study of S-1 Plus Either Irinotecan or Docetaxel for Non-small Cell Lung Cancer Patients Treated with More Than Three Lines of Treatment
- Treatment of Advanced and Metastatic Squamous Non-small Cell Lung Cancer