Yonsei Med J.
2017 Mar;58(2):453-457. 10.3349/ymj.2017.58.2.453.
Sacral Reconstruction with a 3D-Printed Implant after Hemisacrectomy in a Patient with Sacral Osteosarcoma: 1-Year Follow-Up Result
- Affiliations
-
- 1Department of Neurosurgery, Spine and Spinal Cord Institute, Yonsei University College of Medicine, Seoul, Korea. cistern@yuhs.ac
- 2Department of Biomedical Engineering, Yonsei University College of Medicine, Seoul, Korea.
- 3Medyssey Co., Ltd., Uijeongbu, Korea.
- 4Department of Pediatric Neurosurgery, Severance Children's Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Pediatric Hemato-Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- 6Department of Neurosurgery, Guro Cham Teun Teun Hospital, Seoul, Korea.
- KMID: 2427137
- DOI: http://doi.org/10.3349/ymj.2017.58.2.453
Abstract
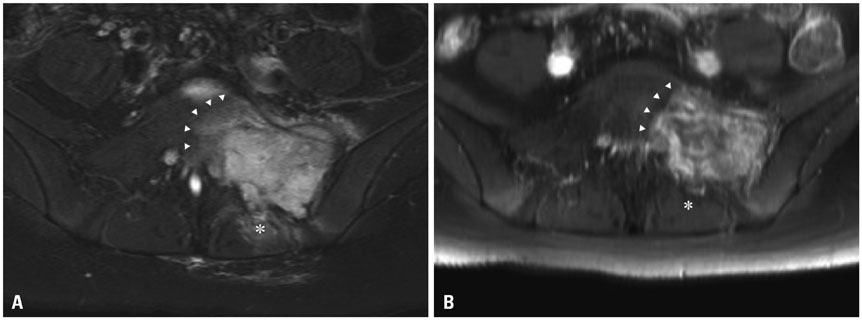
- Pelvic reconstruction after sacral resection is challenging in terms of anatomical complexity, excessive loadbearing, and wide defects. Nevertheless, the technological development of 3D-printed implants enables us to overcome these difficulties. Here, we present a case of sacral osteosarcoma surgically treated with hemisacrectomy and sacral reconstruction using a 3D-printed implant. The implant was printed as a customized titanium prosthesis from a 3D real-sized reconstruction of a patient's CT images. It consisted mostly of a porous mesh and incorporated a dense strut. After 3-months of neoadjuvant chemotherapy, the patient underwent hemisacretomy with preservation of contralateral sacral nerves. The implant was anatomically installed on the defect and fixed with a screw-rod system up to the level of L3. Postoperative pain was significantly low and the patient recovered sufficiently to walk as early as 2 weeks postoperatively. The patient showed left-side foot drop only, without loss of sphincter function. In 1-year follow-up CT, excellent bony fusion was noticed. To our knowledge, this is the first report of a case of hemisacral reconstruction using a custom-made 3D-printed implant. We believe that this technique can be applied to spinal reconstructions after a partial or complete spondylectomy in a wide variety of spinal diseases.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
3D Printer Application for Endoscope-Assisted Spine Surgery Instrument Development: From Prototype Instruments to Patient-Specific 3D Models
Hee-Seok Yang, Jeong-Yoon Park
Yonsei Med J. 2020;61(1):94-99. doi: 10.3349/ymj.2020.61.1.94.Implications of 3-Dimensional Printed Spinal Implants on the Outcomes in Spine Surgery
Brian Fiani, Alexander Newhouse, Alessandra Cathel, Kasra Sarhadi, Marisol Soula
J Korean Neurosurg Soc. 2021;64(4):495-504. doi: 10.3340/jkns.2020.0272.
Reference
-
1. Zileli M, Hoscoskun C, Brastianos P, Sabah D. Surgical treatment of primary sacral tumors: complications associated with sacrectomy. Neurosurg Focus. 2003; 15:E9.
Article2. Fourney DR, Rhines LD, Hentschel SJ, Skibber JM, Wolinsky JP, Weber KL, et al. En bloc resection of primary sacral tumors: classification of surgical approaches and outcome. J Neurosurg Spine. 2005; 3:111–122.
Article3. Zoccali C, Skoch J, Patel A, Walter CM, Maykowski P, Baaj AA. The surgical neurovascular anatomy relating to partial and complete sacral and sacroiliac resections: a cadaveric, anatomic study. Eur Spine J. 2015; 24:1109–1113.
Article4. Mukherjee D, Chaichana KL, Parker SL, Gokaslan ZL, McGirt MJ. Association of surgical resection and survival in patients with malignant primary osseous spinal neoplasms from the Surveillance, Epidemiology, and End Results (SEER) database. Eur Spine J. 2013; 22:1375–1382.
Article5. Li D, Guo W, Tang X, Yang R, Tang S, Qu H, et al. Preservation of the contralateral sacral nerves during hemisacrectomy for sacral malignancies. Eur Spine J. 2014; 23:1933–1939.
Article6. Ozturk AK, Gokaslan ZL, Wolinsky JP. Surgical treatment of sarcomas of the spine. Curr Treat Options Oncol. 2014; 15:482–492.
Article7. Yu B, Zheng Z, Zhuang X, Chen H, Xie D, Luk KD, et al. Biomechanical effects of transverse partial sacrectomy on the sacroiliac joints: an in vitro human cadaveric investigation of the borderline of sacroiliac joint instability. Spine (Phila Pa 1976). 2009; 34:1370–1375.
Article8. Hugate RR Jr, Dickey ID, Phimolsarnti R, Yaszemski MJ, Sim FH. Mechanical effects of partial sacrectomy: when is reconstruction necessary? Clin Orthop Relat Res. 2006; 450:82–88.9. McKnight AJ, Lewis VO, Rhines LD, Hanasono MM. Femur-fibula-fillet of leg chimeric free flap for sacral-pelvic reconstruction. J Plast Reconstr Aesthet Surg. 2013; 66:1784–1787.
Article10. Wang J, Tang Q, Xie X, Yin J, Zhao Z, Li Z, et al. Iliosacral resections of pelvic malignant tumors and reconstruction with nonvascular bilateral fibular autografts. Ann Surg Oncol. 2012; 19:4043–4051.
Article11. Gillis CC, Street JT, Boyd MC, Fisher CG. Pelvic reconstruction after subtotal sacrectomy for sacral chondrosarcoma using cadaveric and vascularized fibula autograft: technical note. J Neurosurg Spine. 2014; 21:623–627.
Article12. Mendel E, Mayerson JL, Nathoo N, Edgar RL, Schmidt C, Miller MJ. Reconstruction of the pelvis and lumbar-pelvic junction using 2 vascularized autologous bone grafts after en bloc resection for an iliosacral chondrosarcoma. J Neurosurg Spine. 2011; 15:168–173.
Article13. Wuisman P, Lieshout O, van Dijk M, van Diest P. Reconstruction after total en bloc sacrectomy for osteosarcoma using a custom-made prosthesis: a technical note. Spine (Phila Pa 1976). 2001; 26:431–439.
Article14. Gokaslan ZL, Romsdahl MM, Kroll SS, Walsh GL, Gillis TA, Wildrick DM, et al. Total sacrectomy and Galveston L-rod reconstruction for malignant neoplasms. Technical note. J Neurosurg. 1997; 87:781–787.
Article15. Newman CB, Keshavarzi S, Aryan HE. En bloc sacrectomy and reconstruction: technique modification for pelvic fixation. Surg Neurol. 2009; 72:752–756.
Article16. Doita M, Harada T, Iguchi T, Sumi M, Sha H, Yoshiya S, et al. Total sacrectomy and reconstruction for sacral tumors. Spine (Phila Pa 1976). 2003; 28:E296–E301.
Article17. Kim JE, Pang J, Christensen JM, Coon D, Zadnik PL, Wolinsky JP, et al. Soft-tissue reconstruction after total en bloc sacrectomy. J Neurosurg Spine. 2015; 22:571–581.
Article18. Girasole G, Muro G, Mintz A, Chertoff J. Transforaminal lumbar interbody fusion rates in patients using a novel titanium implant and demineralized cancellous allograft bone sponge. Int J Spine Surg. 2013; 7:e95–e100.
Article19. Palmquist A, Snis A, Emanuelsson L, Browne M, Thomsen P. Long-term biocompatibility and osseointegration of electron beam melted, free-form-fabricated solid and porous titanium alloy: experimental studies in sheep. J Biomater Appl. 2013; 27:1003–1016.
Article20. Li JP, Li SH, Van Blitterswijk CA, de Groot K. A novel porous Ti6Al4V: characterization and cell attachment. J Biomed Mater Res A. 2005; 73:223–233.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reconstruction of the Cervical Lateral Mass Using 3-Dimensional-Printed Prostheses
- 3D-printed titanium implant with pre-mounted dental implants for mandible reconstruction: a case report
- Fabrication of 3D-Printed Implant for Two-Stage Ear Reconstruction Surgery and Its Clinical Application
- Sacral Nerve Stimulation Through the Sacral Hiatus
- Reply to Commentary on “Sacral Nerves Reconstruction After Surgical Resection of a Large Sacral Chordoma Restores the Urinary and Sexual Function and the Anal Continence”