J Vet Sci.
2018 Nov;19(6):827-834. 10.4142/jvs.2018.19.6.827.
Presurgical assessment of splenic tumors in dogs: a retrospective study of 57 cases (2012–2017)
- Affiliations
-
- 1Department of Veterinary Surgery, College of Veterinary Medicine, Chungnam National University, Daejeon 34134, Korea. jsmok@cnu.ac.kr
- 2Department of Veterinary Diagnostic Imaging, College of Veterinary Medicine, Chungnam National University, Daejeon 34134, Korea.
- KMID: 2427031
- DOI: http://doi.org/10.4142/jvs.2018.19.6.827
Abstract
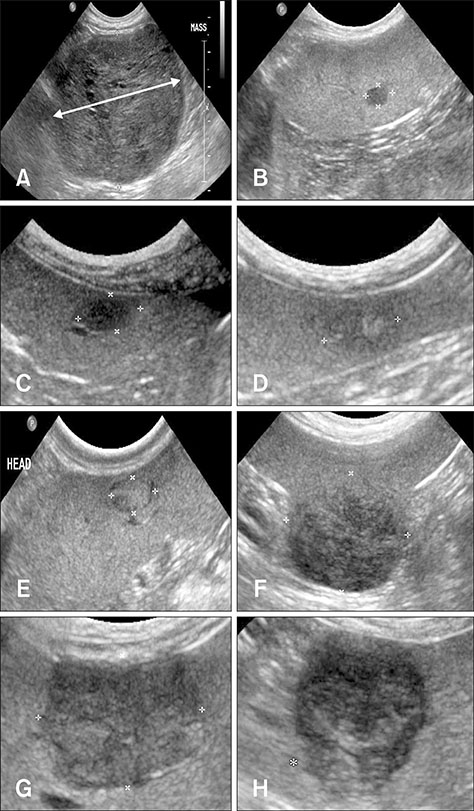
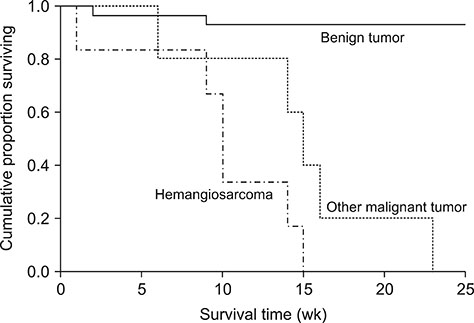
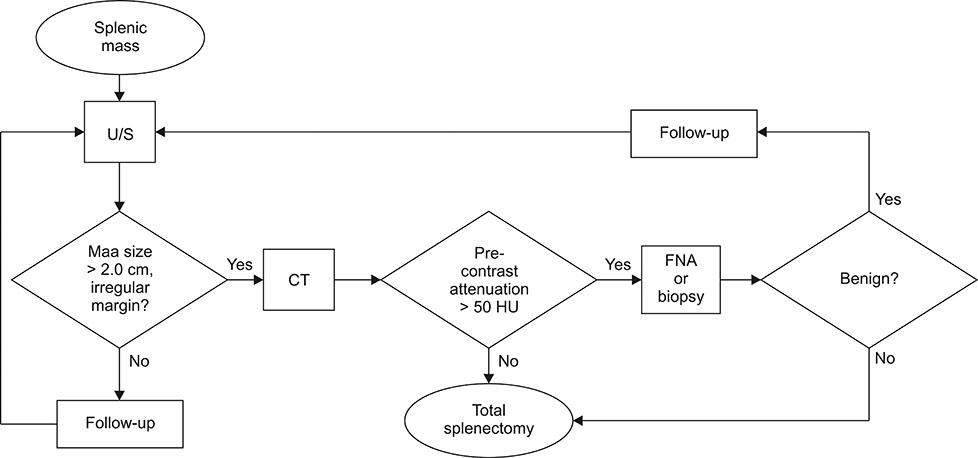
- The purpose of this study was to evaluate the clinical and imaging characteristics of canine splenic tumors and to establish guidelines for the presurgical assessment of splenic tumors in dogs. Fifty-seven dogs that underwent total splenectomy for the treatment of splenic tumors were evaluated by examining medical records, hematologic results, diagnostic imaging results, and histopathologic results. The maximum lesion size from ultrasonography was significantly different between malignant and benign tumors (p = 0.002). There was a correlation between tumor margination and type of splenic tumors (p = 0.045). Precontrast lesion attenuation on computed tomography was significantly different between splenic malignant and benign tumors (p = 0.001). The mean ± SD precontrast lesion attenuation of malignant tumors was 40.3 ± 5.9 Hounsfield units (HU), and for benign tumors, it was 52.8 ± 6.8 HU. In conclusion, some variables of the imaging examination could be used to distinguish the type of splenic tumor. Based on the study results, using a diagnostic flowchart would be effective in increasing the survival rate of patients with splenic malignant tumors. In addition, fine needle aspiration or magnetic resonance imaging prior to surgical exploration and histopathologic examination may be useful in achieving a more accurate diagnosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Ballegeer EA, Forrest LJ, Dickinson RM, Schutten MM, Delaney FA, Young KM. Correlation of ultrasonographic appearance of lesions and cytologic and histologic diagnoses in splenic aspirates from dogs and cats: 32 cases (2002-2005). J Am Vet Med Assoc. 2007; 230:690–696.
Article2. Brown NO, Patnaik AK, MacEwen EG. Canine hemangiosarcoma: retrospective analysis of 104 cases. J Am Vet Med Assoc. 1985; 186:56–58.3. Civardi G, Vallisa D, Bertè R, Giorgio A, Filice C, Caremani M, Caturelli E, Pompili M, De Sio I, Buscarini E, Cavanna L. Ultrasound-guided fine needle biopsy of the spleen: high clinical efficacy and low risk in a multicenter Italian study. Am J Hematol. 2001; 67:93–99.
Article4. Clifford CA, Pretorius ES, Weisse C, Sorenmo KU, Drobatz KJ, Siegelman ES, Solomon JA. Magnetic resonance imaging of focal splenic and hepatic lesions in the dog. J Vet Intern Med. 2004; 18:330–338.
Article5. Corbin EE, Cavanaugh RP, Schwartz P, Zawadzki KI, Donovan T. Splenomegaly in small-breed dogs: 45 cases (2005-2011). J Am Vet Med Assoc. 2017; 250:1148–1154.6. Dane DM, Hsia CC, Wu EY, Hogg RT, Hogg DC, Estrera AS, Johnson RL Jr. Splenectomy impairs diffusive oxygen transport in the lung of dogs. J Appl Physiol (1985). 2006; 101:289–297.
Article7. Fife WD, Samii VF, Drost WT, Mattoon JS, Hoshaw-Woodard S. Comparison between malignant and nonmalignant splenic masses in dogs using contrast-enhanced computed tomography. Vet Radiol Ultrasound. 2004; 45:289–297.
Article8. Hammond TN, Pesillo-Crosby SA. Prevalence of hemangiosarcoma in anemic dogs with a splenic mass and hemoperitoneum requiring a transfusion: 71 cases (2003-2005). J Am Vet Med Assoc. 2008; 232:553–558.
Article9. Hanson JA, Papageorges M, Girard E, Menard M, Hebert P. Ultrasonographic appearance of splenic disease in 101 cats. Vet Radiol Ultrasound. 2001; 42:441–445.
Article10. Ivančić M, Long F, Seiler GS. Contrast harmonic ultrasonography of splenic masses and associated liver nodules in dogs. J Am Vet Med Assoc. 2009; 234:88–94.
Article11. Johnson KA, Powers BE, Withrow SJ, Sheetz MJ, Curtis CR, Wrigley RH. Splenomegaly in dogs. Predictors of neoplasia and survival after splenectomy. J Vet Intern Med. 1989; 3:160–166.12. Jones ID, Lamb CR, Drees R, Priestnall SL, Mantis P. Associations between dual-phase computed tomography features and histopathologic diagnoses in 52 dogs with hepatic or splenic masses. Vet Radiol Ultrasound. 2016; 57:144–153.
Article13. Karakas HM, Demir M, Ozyilmaz F, Cakir B. Primary angiosarcoma of the spleen: in vivo and in vitro MRI findings. Clin Imaging. 2001; 25:192–196.14. Mallinckrodt MJ, Gottfried SD. Mass-to-splenic volume ratio and splenic weight as a percentage of body weight in dogs with malignant and benign splenic masses: 65 cases (2007-2008). J Am Vet Med Assoc. 2011; 239:1325–1327.
Article15. Ng CY, Mills JN. Clinical and haematological features of haemangiosarcoma in dogs. Aust Vet J. 1985; 62:1–4.
Article16. O'Keefe DA, Couto CG. Fine-needle aspiration of the spleen as an aid in the diagnosis of splenomegaly. J Vet Intern Med. 1987; 1:102–109.17. Patel N, Dawe G, Tung K. Ultrasound-guided percutaneous splenic biopsy using an 18-G core biopsy needle: our experience with 52 cases. Br J Radiol. 2015; 88:20150400.
Article18. Ramirez S, Douglass JP, Robertson ID. Ultrasonographic features of canine abdominal malignant histiocytosis. Vet Radiol Ultrasound. 2002; 43:167–170.
Article19. Richardson EF, Brown NO. Hematological and biochemical changes and results of aerobic bacteriological culturing in dogs undergoing splenectomy. J Am Anim Hosp Assoc. 1996; 32:199–210.
Article20. Schwarz LA, Penninck DG, Gliatto J. Canine splenic myelolipomas. Vet Radiol Ultrasound. 2001; 42:347–348.21. Solbiati L, Bossi MC, Bellotti E, Ravetto C, Montali G. Focal lesions in the spleen: sonographic patterns and guided biopsy. AJR Am J Roentgenol. 1983; 140:59–65.
Article22. Spangler WL, Kass PH. Pathologic factors affecting postsplenectomy survival in dogs. J Vet Intern Med. 1997; 11:166–171.
Article23. Wendelburg KM, O'Toole TE, McCobb E, Price LL, Lyons JA, Berg J. Risk factors for perioperative death in dogs undergoing splenectomy for splenic masses: 539 cases (2001-2012). J Am Vet Med Assoc. 2014; 245:1382–1390.
Article24. Wood CA, Moore AS, Gliatto JM, Ablin LA, Berg RJ, Rand WM. Prognosis for dogs with stage I or II splenic hemangiosarcoma treated by splenectomy alone: 32 cases (1991-1993). J Am Anim Hosp Assoc. 1998; 34:417–421.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Splenic smooth muscle tumors in 7 dogs: case reports
- Demographics of dogs and cats with oral tumors presenting to teaching hospitals: 1996–2017
- Evaluation of circulating IGF-I and IGFBP-3 as biomarkers for tumors in dogs
- Assessment of prognostic factors in dogs with mammary gland tumors: 60 cases (2014-2020)
- Mammary gland tumors in three male dogs