J Vet Sci.
2018 Nov;19(6):808-816. 10.4142/jvs.2018.19.6.808.
Combination of berberine and ciprofloxacin reduces multi-resistant Salmonella strain biofilm formation by depressing mRNA expressions of luxS, rpoE, and ompR
- Affiliations
-
- 1College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, China. fangpingliu@126.com
- 2Heilongjiang Key Laboratory for Animal Disease Control and Pharmaceutical Development, Harbin 150030, China.
- 3Harbin Veterinary Research Institute of Chinese Academy of Agricultural Sciences, Harbin 150069, China.
- KMID: 2427029
- DOI: http://doi.org/10.4142/jvs.2018.19.6.808
Abstract
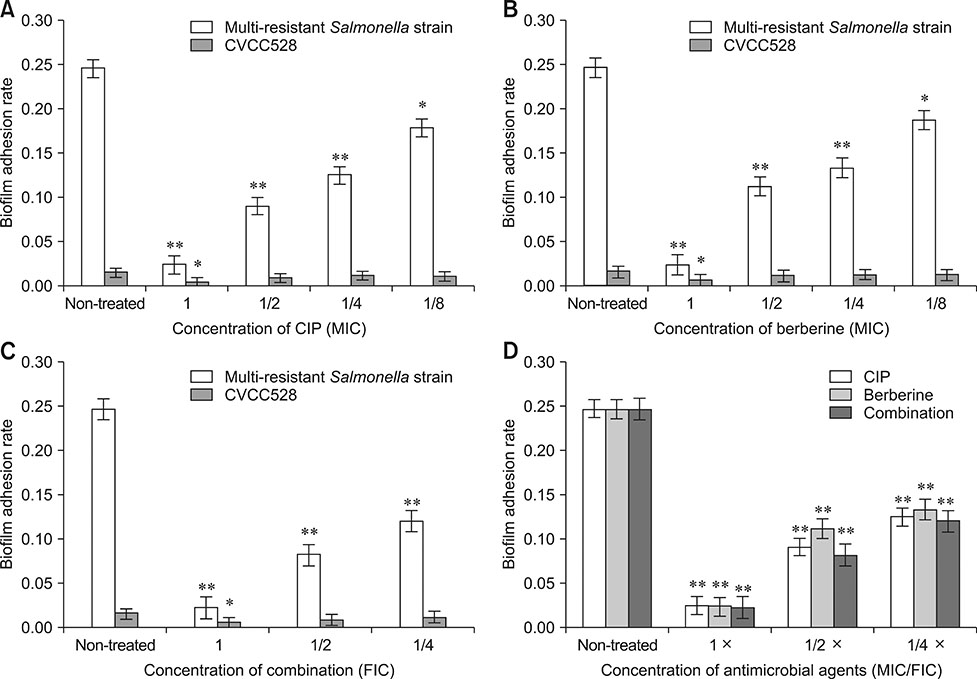
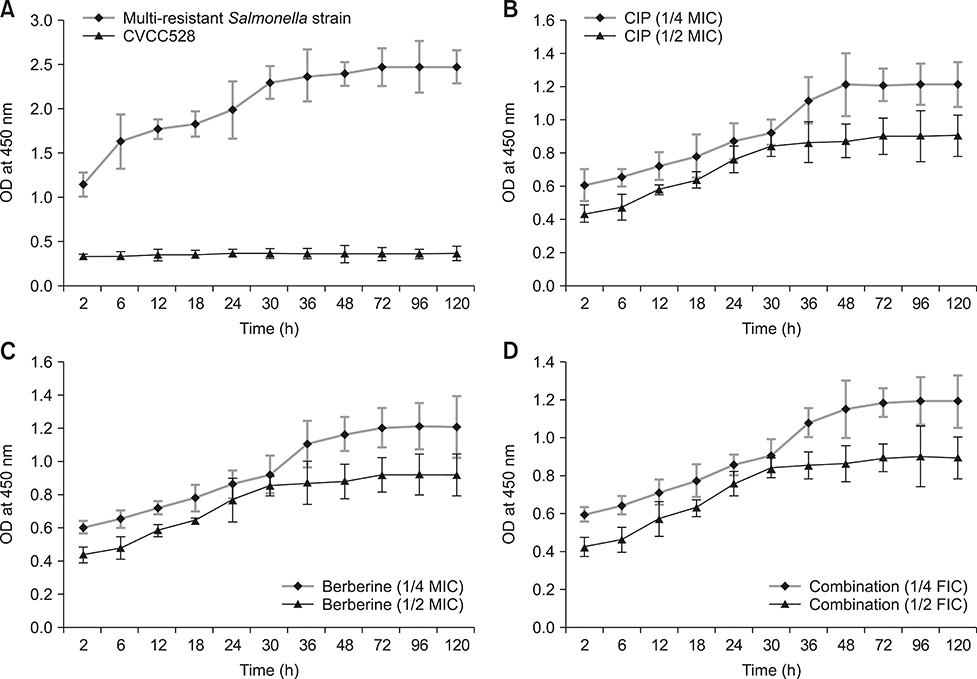
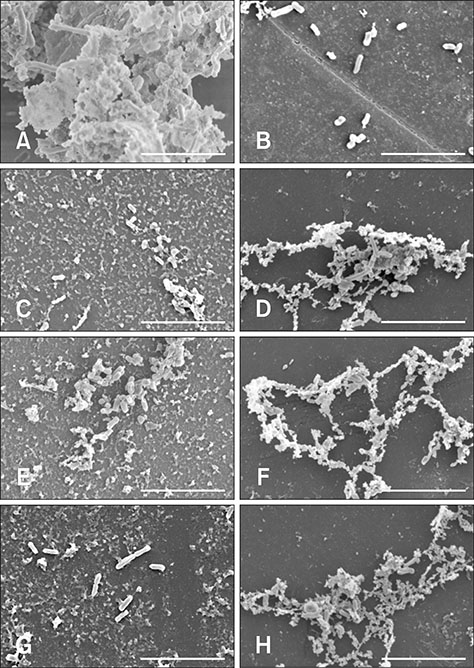
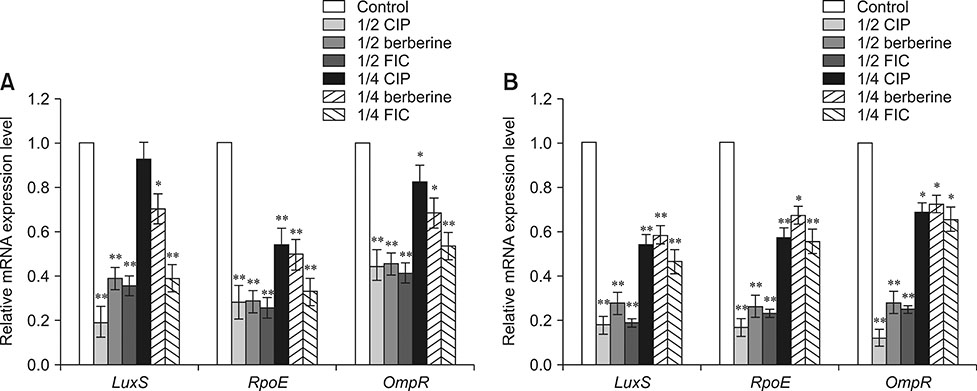
- Bacterial biofilms have been demonstrated to be closely related to clinical infections and contribute to drug resistance. Berberine, which is the main component of Coptis chinensis, has been reported to have efficient antibacterial activity. This study aimed to investigate the potential effect of a combination of berberine with ciprofloxacin (CIP) to inhibit Salmonella biofilm formation and its effect on expressions of related genes (rpoE, luxS, and ompR). The fractional inhibitory concentration (FIC) index of the combination of berberine with CIP is 0.75 showing a synergistic antibacterial effect. The biofilm's adhesion rate and growth curve showed that the multi-resistant Salmonella strain had the potential to form a biofilm relative to that of strain CVCC528, and the antibiofilm effects were in a dose-dependent manner. Biofilm microstructures were rarely observed at 1/2 × MIC/FIC concentrations (MIC, minimal inhibition concentration), and the combination had a stronger antibiofilm effect than each of the antimicrobial agents used alone at 1/4 × FIC concentration. LuxS, rpoE, and ompR mRNA expressions were significantly repressed (p < 0.01) at 1/2 × MIC/FIC concentrations, and the berberine and CIP combination repressed mRNA expressions more strongly at the 1/4 × FIC concentration. The results indicate that the combination of berberine and CIP has a synergistic effect and is effective in inhibiting Salmonella biofilm formation via repression of luxS, rpoE, and ompR mRNA expressions.
Keyword
MeSH Terms
Figure
Reference
-
1. Abdallah M, Benoliel C, Drider D, Dhulster P, Chihib NE. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch Microbiol. 2014; 196:453–472.
Article2. Bai L, Zhao J, Gan X, Wang J, Zhang X, Cui S, Xia S, Hu Y, Yan S, Wang J, Li F, Fanning S, Xu J. Emergence and diversity of Salmonella enterica serovar Indiana isolates with concurrent resistance to ciprofloxacin and cefotaxime from patients and food-producing animals in China. Antimicrob Agents Chemother. 2016; 60:3365–3371.
Article3. Castelijn GA, Parabirsing JA, Zwietering MH, Moezelaar R, Abee T. Surface behaviour of S. Typhimurium, S. Derby, S. Brandenburg and S. Infantis. Vet Microbiol. 2013; 161:305–314.
Article4. Castelijn GA, van der Veen S, Zwietering MH, Moezelaar R, Abee T. Diversity in biofilm formation and production of curli fimbriae and cellulose of Salmonella Typhimurium strains of different origin in high and low nutrient medium. Biofouling. 2012; 28:51–63.
Article5. Chakraborty S, Mizusaki H, Kenney LJ. A FRET-based DNA biosensor tracks OmpR-dependent acidification of Salmonella during macrophage infection. PLoS Biol. 2015; 13:e1002116.6. Choi JS, Ali MY, Jung HA, Oh SH, Choi RJ, Kim EJ. Protein tyrosine phosphatase 1B inhibitory activity of alkaloids from Rhizoma Coptidis and their molecular docking studies. J Ethnopharmacol. 2015; 171:28–36.
Article7. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. Wayne: CLSI;2018. CLSI Supplement M100.8. Cogan TA, Humphrey TJ. The rise and fall of Salmonella Enteritidis in the UK. J Appl Microbiol. 2003; 94:114S–119S.9. Dai C, Wang J, Kong W, Xiao X, Peng C, Zhao Y, Jin C. Investigation on the antibacterial activity of Coptis chinensis Franch and its components compatibility by microcalorimetry. Acta Chim Sinica. 2010; 68:936–940.10. Garmendia J, Beuzón CR, Ruiz-Albert J, Holden DW. The roles of SsrA-SsrB and OmpR-EnvZ in the regulation of genes encoding the Salmonella typhimurium SPI-2 type III secretion system. Microbiology. 2003; 149:2385–2396.
Article11. Jeong HH, Jeong SG, Park A, Jang SC, Hong SG, Lee CS. Effect of temperature on biofilm formation by Antarctic marine bacteria in a microfluidic device. Anal Biochem. 2014; 446:90–95.
Article12. Kim JH, Yu D, Eom SH, Kim SH, Oh J, Jung WK, Kim YM. Synergistic antibacterial effects of chitosan-caffeic acid conjugate against antibiotic-resistant acne-related bacteria. Mar Drugs. 2017; 15:pii: E167.
Article13. Li J, Overall CC, Nakayasu ES, Kidwai AS, Jones MB, Johnson RC, Nguyen NT, McDermott JE, Ansong C, Heffron F, Cambronne ED, Adkins JN. Analysis of the Salmonella regulatory network suggests involvement of SsrB and H-NS in σE-regulated SPI-2 gene expression. Front Microbiol. 2015; 6:27.
Article14. Li YH, Zhou YH, Ren YZ, Xu CG, Liu X, Liu B, Chen JQ, Ding WY, Zhao YL, Yang YB, Wang S, Liu D. Inhibition of Streptococcus suis adhesion and biofilm formation in vitro by water extracts of Rhizoma Coptidis. Front Pharmacol. 2018; 9:371.
Article15. Ling H, Kang A, Tan MH, Qi X, Chang MW. The absence of the luxS gene increases swimming motility and flagella synthesis in Escherichia coli K12. Biochem Biophys Res Commun. 2010; 401:521–526.
Article16. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001; 25:402–408.
Article17. Marshall BM, Levy SB. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev. 2011; 24:718–733.
Article18. Norden CW, Wentzel H, Keleti E. Comparison of techniques for measurement of in vitro antibiotic synergism. J Infect Dis. 1979; 140:629–633.
Article19. Ong ES, Woo SO, Yong YL. Pressurized liquid extraction of berberine and aristolochic acids in medicinal plants. J Chromatogr A. 2000; 904:57–64.
Article20. Rachid S, Ohlsen K, Witte W, Hacker J, Ziebuhr W. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob Agents Chemother. 2000; 44:3357–3363.
Article21. Rao RS, Karthika RU, Singh SP, Shashikala P, Kanungo R, Jayachandran S, Prashanth K. Correlation between biofilm production and multiple drug resistance in imipenem resistant clinical isolates of Acinetobacter baumannii. Indian J Med Microbiol. 2008; 26:333–337.
Article22. Sela S, Frank S, Belausov E, Pinto R. A mutation in the luxS gene influences Listeria monocytogenes biofilm formation. Appl Environ Microbiol. 2006; 72:5653–5658.
Article23. Sharma M, Manoharlal R, Negi AS, Prasad R. Synergistic anticandidal activity of pure polyphenol curcumin I in combination with azoles and polyenes generates reactive oxygen species leading to apoptosis. FEMS Yeast Res. 2010; 10:570–578.
Article24. Steenackers HP, Janssens JC, Levin J, Voet A, De Maeyer M, De Vos DE, Vanderleyden J, De Keersmaecker SJ. Inhibition of salmonella biofilm formation: a sustainable alternative in the production of safe and healthy food. Commun Agric Appl Biol Sci. 2008; 73:71–76.25. Sun Y, Dai M, Hao H, Wang Y, Huang L, Almofti YA, Liu Z, Yuan Z. The role of RamA on the development of ciprofloxacin resistance in Salmonella enterica serovar Typhimurium. PLoS One. 2011; 6:e23471.26. Tobin DM, Vary JC Jr, Ray JP, Walsh GS, Dunstan SJ, Bang ND, Hagge DA, Khadge S, King MC, Hawn TR, Moens CB, Ramakrishnan L. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010; 140:717–730.
Article27. Wang S, Yang Y, Zhao Y, Zhao H, Bai J, Chen J, Zhou Y, Wang C, Li Y. Sub-MIC tylosin inhibits Streptococcus suis biofilm formation and results in differential protein expression. Front Microbiol. 2016; 7:384.
Article28. Yang Y, Ye XL, Li XG, Zhen J, Zhang B, Yuan L. Synthesis and antimicrobial activity of 8-alkylberberine derivatives with a long aliphatic chain. Planta Med. 2007; 73:602–604.
Article29. Yang YB, Wang S, Wang C, Huang QY, Bai JW, Chen JQ, Chen XY, Li YH. Emodin affects biofilm formation and expression of virulence factors in Streptococcus suis ATCC700794. Arch Microbiol. 2015; 197:1173–1180.
Article30. Yu HH, Kim KJ, Cha JD, Kim HK, Lee YE, Choi NY, You YO. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J Med Food. 2005; 8:454–461.
Article31. Zhao H, Zhou S, Zhang M, Feng J, Wang S, Wang D, Geng Y, Wang X. An in vitro AChE inhibition assay combined with UF-HPLC-ESI-Q-TOF/MS approach for screening and characterizing of AChE inhibitors from roots of Coptis chinensis Franch. J Pharm Biomed Anal. 2016; 120:235–240.
Article32. Zogaj X, Bokranz W, Nimtz M, Römling U. Production of cellulose and curli fimbriae by members of the family Enterobacteriaceae isolated from the human gastrointestinal tract. Infect Immun. 2003; 71:4151–4158.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Time Kill Studies of Antibiotics against a Nalidixic Acid Resistant Salmonella enterica serotype Typhi
- A Virulent Salmonella enterica Serovar Enteritidis Phage SE2 with a Strong Bacteriolytic Activity of Planktonic and Biofilmed Cells
- A Case of Typhoid Fever to Failed Ciprofloxacin, Infected in Korea
- A Case of Multidrug-Resistant Salmonella enterica Serovar Typhi Treated with a Bench to Bedside Approach
- Transcriptional Analysis of the iagB within Salmonella Pathogenicity Island 1 (SPI1)