J Vet Sci.
2018 Nov;19(6):744-749. 10.4142/jvs.2018.19.6.744.
Dapsone modulates lipopolysaccharide-activated bone marrow cells by inducing cell death and down-regulating tumor necrosis factor-α production
- Affiliations
-
- 1College of Veterinary Medicine, Jeju National University, Jeju 63243, Korea. jooh@jejunu.ac.kr
- 2Veterinary Medical Research Institute, Jeju National University, Jeju 63243, Korea.
- KMID: 2427022
- DOI: http://doi.org/10.4142/jvs.2018.19.6.744
Abstract
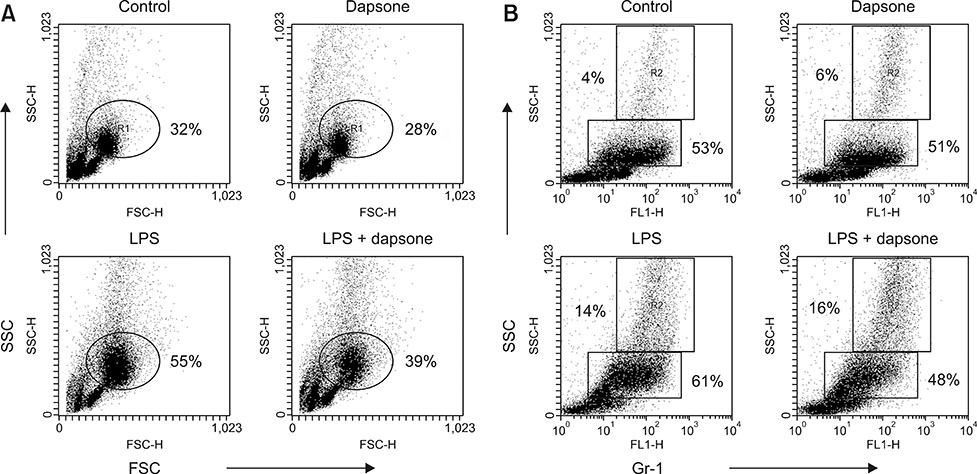
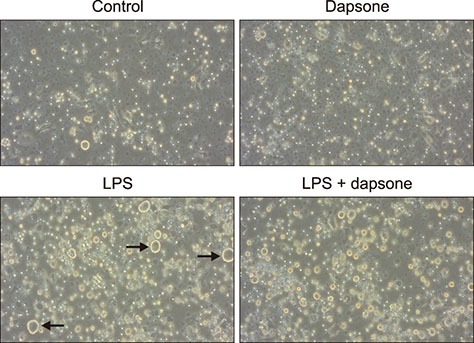
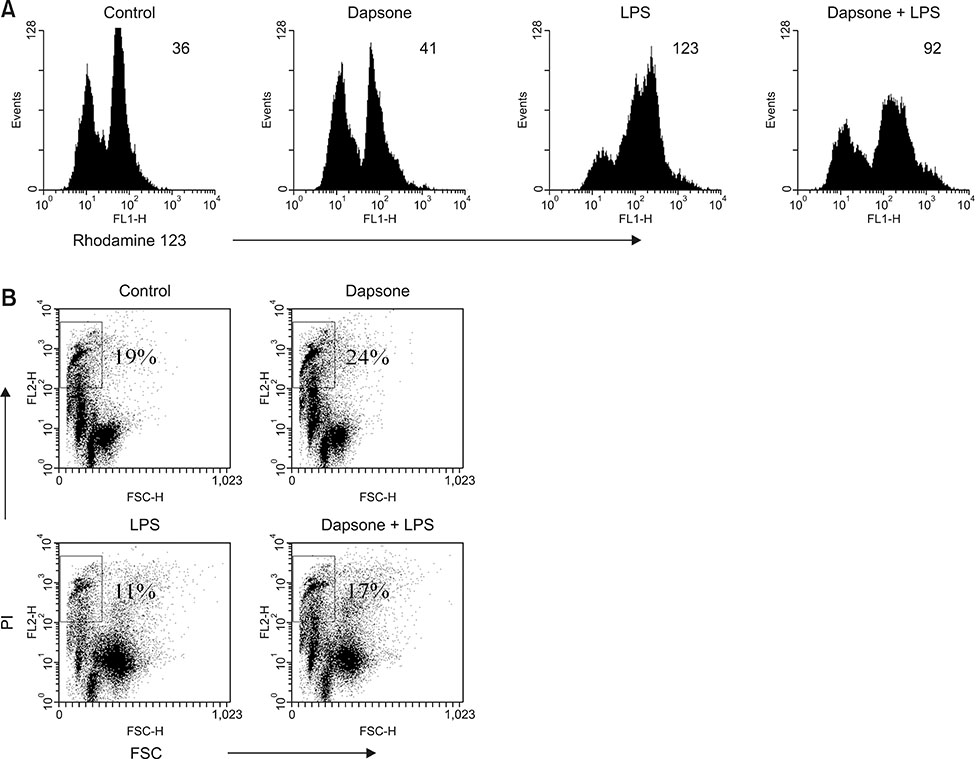
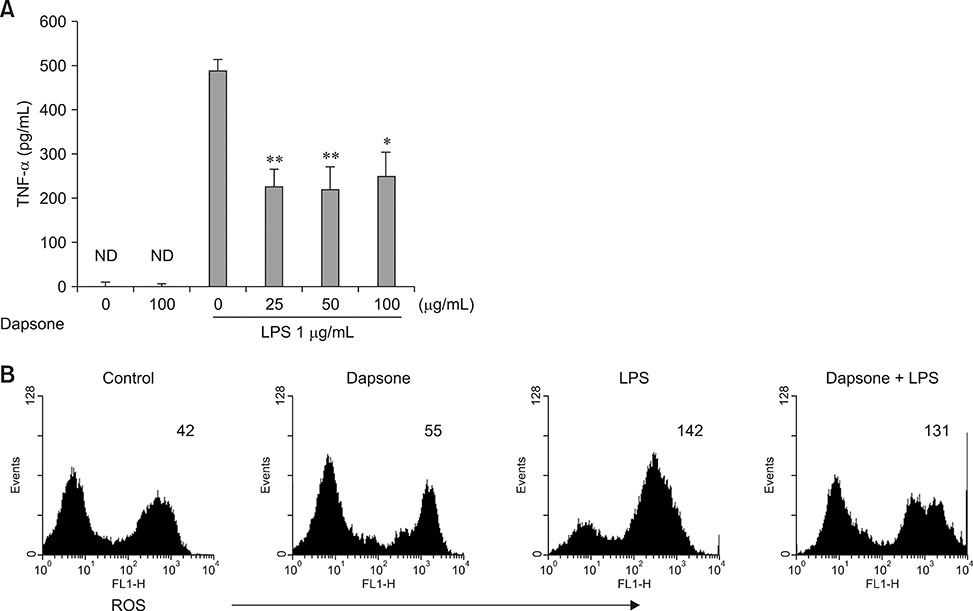
- Dapsone, an antibiotic, has been used to cure leprosy. It has been reported that dapsone has anti-inflammatory activity in hosts; however, the anti-inflammatory mechanism of dapsone has not been fully elucidated. The present study investigated the anti-inflammatory effects of dapsone on bone marrow cells (BMs), especially upon exposure to lipopolysaccharide (LPS). We treated BMs with LPS and dapsone, and the treated cells underwent cellular activity assay, flow cytometry analysis, cytokine production assessment, and reactive oxygen species assay. LPS distinctly activated BMs with several characteristics including high cellular activity, granulocyte changes, and tumor necrosis factor alpha (TNF-α) production increases. Interestingly, dapsone modulated the inflammatory cells, including granulocytes in LPS-treated BMs, by inducing cell death. While the percentage of Gr-1 positive cells was 57% in control cells, LPS increased that to 75%, and LPS plus dapsone decreased it to 64%. Furthermore, dapsone decreased the mitochondrial membrane potential of LPS-treated BMs. At a low concentration (25 µg/mL), dapsone significantly decreased the production of TNF-α in LPS-treated BMs by 54%. This study confirmed that dapsone has anti-inflammatory effects on LPS-mediated inflammation via modulation of the number and function of inflammatory cells, providing new and useful information for clinicians and researchers.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Evaluation of the effects of disulfiram, an alcohol-aversive agent with anti-cancer activity, on mouse bone marrow cells
Seo-Ro Park, Hong-Gu Joo
Korean J Physiol Pharmacol. 2022;26(3):157-164. doi: 10.4196/kjpp.2022.26.3.157.
Reference
-
1. Abe M, Shimizu A, Yokoyama Y, Takeuchi Y, Ishikawa O. A possible inhibitory action of diaminodiphenyl sulfone on tumour necrosis factor-α production from activated mononuclear cells on cutaneous lupus erythematosus. Clin Exp Dermatol. 2008; 33:759–763.
Article2. Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med. 2007; 146:657–665.
Article3. Aratani Y. Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys. 2018; 640:47–52.
Article4. Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975; 72:3666–3670.
Article5. Chan CC, Rodger IW. Selective cyclooxygenase-2 inhibitors as potential therapeutic agents for inflammatory diseases. Adv Exp Med Biol. 1997; 407:157–161.
Article6. Coleman MD. Dapsone-mediated agranulocytosis: risks, possible mechanisms and prevention. Toxicology. 2001; 162:53–60.
Article7. Jang JY, Moon SY, Joo HG. Differential effects of fucoidans with low and high molecular weight on the viability and function of spleen cells. Food Chem Toxicol. 2014; 68:234–238.
Article8. Kanoh S, Tanabe T, Rubin BK. Dapsone inhibits IL-8 secretion from human bronchial epithelial cells stimulated with lipopolysaccharide and resolves airway inflammation in the ferret. Chest. 2011; 140:980–990.
Article9. Kettle AJ, Winterbourn CC. Mechanism of inhibition of myeloperoxidase by anti-inflammatory drugs. Biochem Pharmacol. 1991; 41:1485–1492.
Article10. Kim HJ, Kim MH, Byon YY, Park JW, Jee Y, Joo HG. Radioprotective effects of an acidic polysaccharide of Panax ginseng on bone marrow cells. J Vet Sci. 2007; 8:39–44.
Article11. Matsukawa A, Yoshinaga M. Sequential generation of cytokines during the initiative phase of inflammation, with reference to neutrophils. Inflamm Res. 1998; 47:Suppl 3. S137–S144.
Article12. Perera PY, Mayadas TN, Takeuchi O, Akira S, Zaks-Zilberman M, Goyert SM, Vogel SN. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J Immunol. 2001; 166:574–581.
Article13. Polisson R. Nonsteroidal anti-inflammatory drugs: practical and theoretical considerations in their selection. Am J Med. 1996; 100:31S–36S.
Article14. Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002; 71:635–700.
Article15. Ramachandran G. Gram-positive and gram-negative bacterial toxins in sepsis: a brief review. Virulence. 2014; 5:213–218.
Article16. Wozel G, Blasum C. Dapsone in dermatology and beyond. Arch Dermatol Res. 2014; 306:103–124.
Article17. Zhu YI, Stiller MJ. Dapsone and sulfones in dermatology: overview and update. J Am Acad Dermatol. 2001; 45:420–434.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-inflammatory effects of 4,4'-diaminodiphenyl sulfone (dapsone) in lipopolysaccharide-treated spleen cells: selective inhibition of inflammation-related cytokines
- Modulatory action of enrofloxacin in lipopolysaccharide-induced hyper-activated mouse spleen cells
- Stimulatory effects of Bordetella bronchiseptica antigen on bone marrow cells and immune memory responses
- Regulation of Adhesion Molecule Expression and Stromal Cell-Derived Factor-1 Production in Human Bone Marrow Cells by Interferon-gamma, Tumor Necrosis Factor-alpha, and Transforming Growth Factor-beta1: Implications in Bone Marrow Homing of Hematopoietic Cells
- Cytotoxic Effects of Tumor Necrosis Factor-related Apoptosis-inducing Ligand (TRAIL)and its Molecular Mechanism in Human Gastric Cancer Cells