J Vet Sci.
2018 Nov;19(6):735-743. 10.4142/jvs.2018.19.6.735.
TGF-β1 upregulates the expression of hyaluronan synthase 2 and hyaluronan synthesis in culture models of equine articular chondrocytes
- Affiliations
-
- 1Thailand Excellence Center for Tissue Engineering and Stem Cells, Department of Biochemistry, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand.
- 2Equine Clinic, Department of Companion Animal and Wildlife Clinic, Faculty of Veterinary Medicine, Chiang Mai University, Chiang Mai 50100, Thailand. makhaboocha@gmail.com
- KMID: 2427021
- DOI: http://doi.org/10.4142/jvs.2018.19.6.735
Abstract
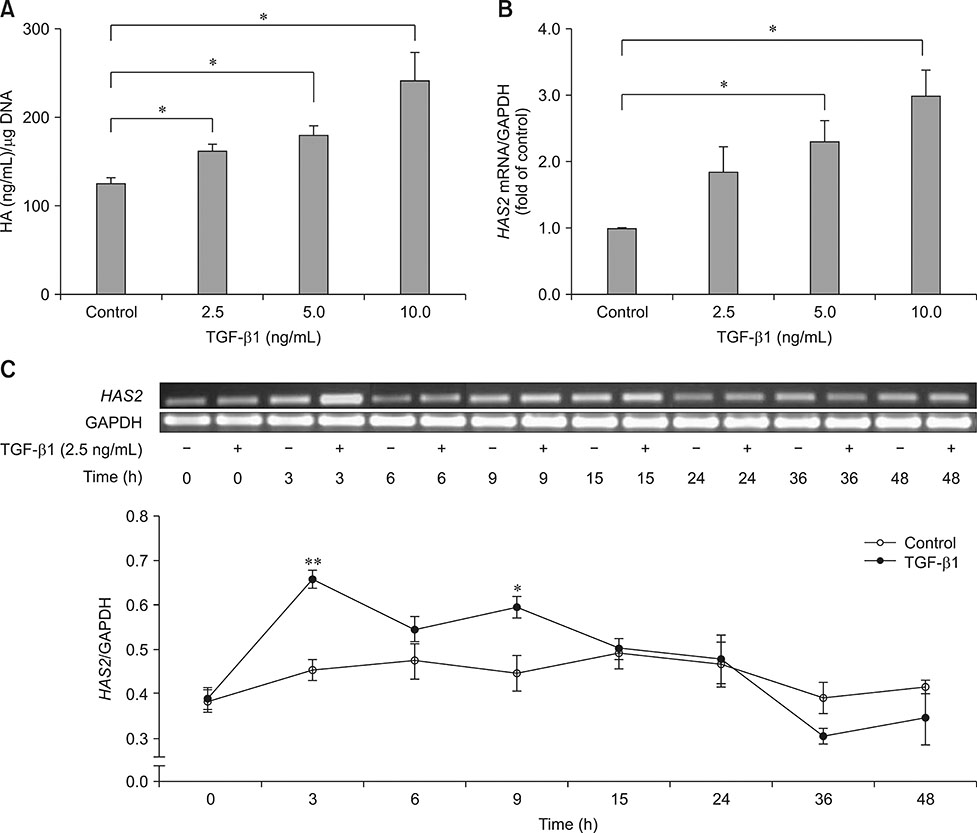
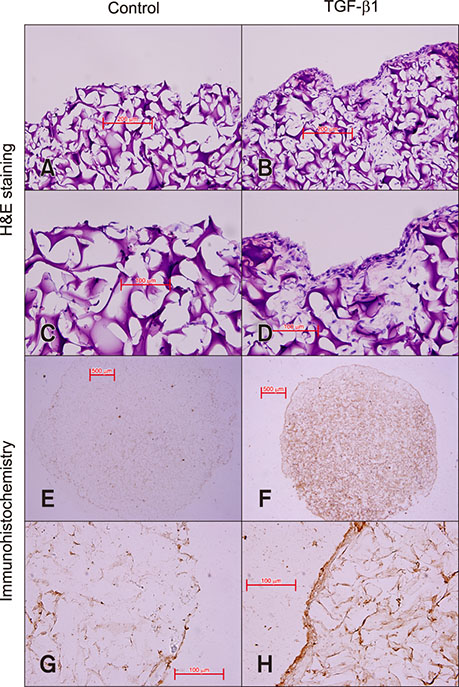
- We investigated the effect of transforming growth factor beta 1 (TGF-β1) on equine hyaluronan synthase 2 (HAS2) gene expression and hyaluronan (HA) synthesis in culture models of articular chondrocytes. Equine chondrocytes were treated with TGF-β1 at different concentrations and times in monolayer cultures. In three-dimensional cultures, chondrocyte-seeded gelatin scaffolds were cultured in chondrogenic media containing 10 ng/mL of TGF-β1. The amounts of HA in conditioned media and in scaffolds were determined by enzyme-linked immunosorbent assays. HAS2 mRNA expression was analyzed by semi-quantitative reverse transcription polymerase chain reaction. The uronic acid content and DNA content of the scaffolds were measured by using colorimetric and Hoechst 33258 assays, respectively. Cell proliferation was evaluated by using the alamarBlue assay. Scanning electron microscopy (SEM), histology, and immunohistochemistry were used for microscopic analysis of the samples. The upregulation of HAS2 mRNA levels by TGF-β1 stimulation was dose and time dependent. TGF-β1 was shown to enhance HA and uronic acid content in the scaffolds. Cell proliferation and DNA content were significantly lower in TGF-β1 treatments. SEM and histological results revealed the formation of a cartilaginous-like extracellular matrix in the TGF-β1-treated scaffolds. Together, our results suggest that TGF-β1 has a stimulatory effect on equine chondrocytes, enhancing HA synthesis and promoting cartilage matrix generation.
MeSH Terms
-
Bisbenzimidazole
Cartilage
Cell Proliferation
Chondrocytes*
Culture Media, Conditioned
DNA
Enzyme-Linked Immunosorbent Assay
Extracellular Matrix
Gelatin
Gene Expression
Horses
Hyaluronic Acid*
Immunohistochemistry
Microscopy, Electron, Scanning
Polymerase Chain Reaction
Reverse Transcription
RNA, Messenger
Transforming Growth Factor beta
Transforming Growth Factors
Up-Regulation
Bisbenzimidazole
Culture Media, Conditioned
DNA
Gelatin
Hyaluronic Acid
RNA, Messenger
Transforming Growth Factor beta
Transforming Growth Factors
Figure
Reference
-
1. Andrejevic K, Backstein D, Kandel R. TGF-beta 3 signaling in redifferentiating passaged human articular chondrocytes. Osteoarthritis Cartilage. 2017; 25:S168–S169.
Article2. Arai K, Kasashima Y, Kobayashi A, Kuwano A, Yoshihara T. TGF-β alters collagen XII and XIV mRNA levels in cultured equine tenocytes. Matrix Biol. 2002; 21:243–250.
Article3. Balazs EA, Watson D, Duff IF, Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis Rheum. 1967; 10:357–376.
Article4. Blanco FJ, Geng Y, Lotz M. Differentiation-dependent effects of IL-1 and TGF-beta on human articular chondrocyte proliferation are related to inducible nitric oxide synthase expression. J Immunol. 1995; 154:4018–4026.5. Chaipinyo K, Oakes BW, van Damme MP. Effects of growth factors on cell proliferation and matrix synthesis of low-density, primary bovine chondrocytes cultured in collagen I gels. J Orthop Res. 2002; 20:1070–1078.
Article6. de Haart M, Marijnissen WJ, van Osch GJ, Verhaar JA. Optimization of chondrocyte expansion in culture. Effect of TGF beta-2, bFGF and L-ascorbic acid on bovine articular chondrocytes. Acta Orthop Scand. 1999; 70:55–61.
Article7. Dische Z. A new specific color reaction of hexuronic acids. J Biol Chem. 1947; 167:189–198.
Article8. Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014; 12:207–218.
Article9. Fortier LA, Nixon AJ, Mohammed HO, Lust G. Altered biological activity of equine chondrocytes cultured in a three-dimensional fibrin matrix and supplemented with transforming growth factor beta-1. Am J Vet Res. 1997; 58:66–70.10. Fukuhira Y, Kaneko H, Yamaga M, Tanaka M, Yamamoto S, Shimomura M. Effect of honeycomb-patterned structure on chondrocyte behavior in vitro. Colloids Surf A Physicochem Eng Asp. 2008; 313-314:520–525.
Article11. Glansbeek HL, van der Kraan PM, Vitters EL, van den Berg WB. Correlation of the size of type II transforming growth factor beta (TGF-beta) receptor with TGF-beta responses of isolated bovine articular chondrocytes. Ann Rheum Dis. 1993; 52:812–816.
Article12. Gordon KJ, Blobe GC. Role of transforming growth factor-β superfamily signaling pathways in human disease. Biochim Biophys Acta. 2008; 1782:197–228.
Article13. Gründer T, Gaissmaier C, Fritz J, Stoop R, Hortschansky P, Mollenhauer J, Aicher WK. Bone morphogenetic protein (BMP)-2 enhances the expression of type II collagen and aggrecan in chondrocytes embedded in alginate beads. Osteoarthritis Cartilage. 2004; 12:559–567.
Article14. Guo N, Li X, Mann MM, Funderburgh ML, Du Y, Funderburgh JL. Hyaluronan synthesis mediates the fibrotic response of keratocytes to transforming growth factor β. J Biol Chem. 2010; 285:32012–32019.
Article15. Hanprasertpong N, Teekachunhatean S, Chaiwongsa R, Ongchai S, Kunanusorn P, Sangdee C, Panthong A, Bunteang S, Nathasaen N, Reutrakul V. Analgesic, anti-inflammatory, and chondroprotective activities of Cryptolepis buchanani extract: in vitro and in vivo studies. Biomed Res Int. 2014; 2014:978582.16. Haubeck HD, Kock R, Fischer DC, Van de Leur E, Hoffmeister K, Greiling H. Transforming growth factor β1, a major stimulator of hyaluronan synthesis in human synovial lining cells. Arthritis Rheum. 1995; 38:669–677.
Article17. Hiscock DRR, Caterson B, Flannery CR. Expression of hyaluronan synthases in articular cartilage. Osteoarthritis Cartilage. 2000; 8:120–126.
Article18. Hwang NS, Kim MS, Sampattavanich S, Baek JH, Zhang Z, Elisseeff J. Effects of three-dimensional culture and growth factors on the chondrogenic differentiation of murine embryonic stem cells. Stem Cells. 2006; 24:284–291.
Article19. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998; 238:265–272.
Article20. Kim YJ, Sah RL, Doong JY, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988; 174:168–176.
Article21. Li Y, Toole BP, Dealy CN, Kosher RA. Hyaluronan in limb morphogenesis. Dev Biol. 2007; 305:411–420.
Article22. Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998; 4:415–428.
Article23. Pruksakorn D, Khamwaen N, Pothacharoen P, Arpornchayanon O, Rojanasthien S, Kongtawelert P. Chondrogenic properties of primary human chondrocytes culture in hyaluronic acid treated gelatin scaffold. J Med Assoc Thai. 2009; 92:483–490.24. Recklies AD, White C, Melching L, Roughley PJ. Differential regulation and expression of hyaluronan synthases in human articular chondrocytes, synovial cells and osteosarcoma cells. Biochem J. 2001; 354:17–24.
Article25. Schulz T, Schumacher U, Prehm P. Hyaluronan export by the ABC transporter MRP5 and its modulation by intracellular cGMP. J Biol Chem. 2007; 282:20999–21004.
Article26. Song JJ, Aswad R, Kanaan RA, Rico MC, Owen TA, Barbe MF, Safadi FF, Popoff SN. Connective tissue growth factor (CTGF) acts as a downstream mediator of TGF-β1 to induce mesenchymal cell condensation. J Cell Physiol. 2007; 210:398–410.
Article27. Spicer AP, McDonald JA. Characterization and molecular evolution of a vertebrate hyaluronan synthase gene family. J Biol Chem. 1998; 273:1923–1932.
Article28. Spicer AP, Olson JS, McDonald JA. Molecular cloning and characterization of a cDNA encoding the third putative mammalian hyaluronan synthase. J Biol Chem. 1997; 272:8957–8961.
Article29. Stock AE, Bouchard N, Brown K, Spicer AP, Underhill CB, Doré M, Sirois J. Induction of hyaluronan synthase 2 by human chorionic gonadotropin in mural granulosa cells of equine preovulatory follicles. Endocrinology. 2002; 143:4375–4384.
Article30. Sugiyama Y, Shimada A, Sayo T, Sakai S, Inoue S. Putative hyaluronan synthase mRNA are expressed in mouse skin and TGF-β upregulates their expression in cultured human skin cells. J Invest Dermatol. 1998; 110:116–121.
Article31. Tangyuenyong S, Viriyakhasem N, Peansukmanee S, Kongtawelert P, Ongchai S. Andrographolide exerts chondroprotective activity in equine cartilage explant and suppresses interleukin-1β-induced MMP-2 expression in equine chondrocyte culture. Int Sch Res Notices. 2014; 2014:464136.
Article32. Tanimoto K, Suzuki A, Ohno S, Honda K, Tanaka N, Doi T, Yoneno K, Ohno-Nakahara M, Nakatani Y, Ueki M, Tanne K. Effects of TGF-β on hyaluronan anabolism in fibroblasts derived from the synovial membrane of the rabbit temporomandibular joint. J Dent Res. 2004; 83:40–44.
Article33. ten Dijke P, Hill CS. New insights into TGF-β-Smad signalling. Trends Biochem Sci. 2004; 29:265–273.
Article34. Thompson CC, Clegg PD, Carter SD. Differential regulation of gelatinases by transforming growth factor beta-1 in normal equine chondrocytes. Osteoarthritis Cartilage. 2001; 9:325–331.
Article35. Viriyakhasem N, Khuajan S, Kongtawelert P, Panthong A, Ongchai S, Reutrakul V. In vitro model of hyaluronan synthase gene expression associated with lipopolysaccharide-induced inflammation in SW982 cell line. In Vitro Cell Dev Biol Anim. 2014; 50:787–791.
Article36. Watts EJ, Rose MT. Platelet-derived growth factor acts via both the Rho-kinase and p38 signaling enzymes to stimulate contraction in an in vitro model of equine wound healing. Domest Anim Endocrinol. 2010; 38:253–259.
Article37. Wu H, Wan Y, Cao X, Wu Q. Proliferation of chondrocytes on porous poly(DL-lactide)/chitosan scaffolds. Acta Biomater. 2008; 4:76–87.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Hyaluronan on Proliferation and Differentiation Cultured Chondrocyte
- Effect of Carboxy Methyl Chitosan on Experimental Osteoarthritis in a Rabbit Knee: Carboxy methyl chitosan vs. Hyaluronan
- Effects of human serum and TGF-beta on proliferation and redifferentiation of human articular chondrocytes
- An Experimental Study about the Influence of TGF-β1 upon Fracture Callus Formation
- The Effect of 3-Dimensional Microcarrier Culture on Chondrocyte Proliferation and Matrix Production