Investig Clin Urol.
2016 Jun;57(Suppl 1):S4-S13. 10.4111/icu.2016.57.S1.S4.
Active surveillance for nonmuscle invasive bladder cancer
- Affiliations
-
- 1Department of Urology, Nara Medical University, Kashihara, Nara, Japan. hiraoyos@gmail.com
- 2Department of Urology, Osaka Gyoumeikan Hospital, Konohana-ku, Osaka, Japan.
- KMID: 2426512
- DOI: http://doi.org/10.4111/icu.2016.57.S1.S4
Abstract
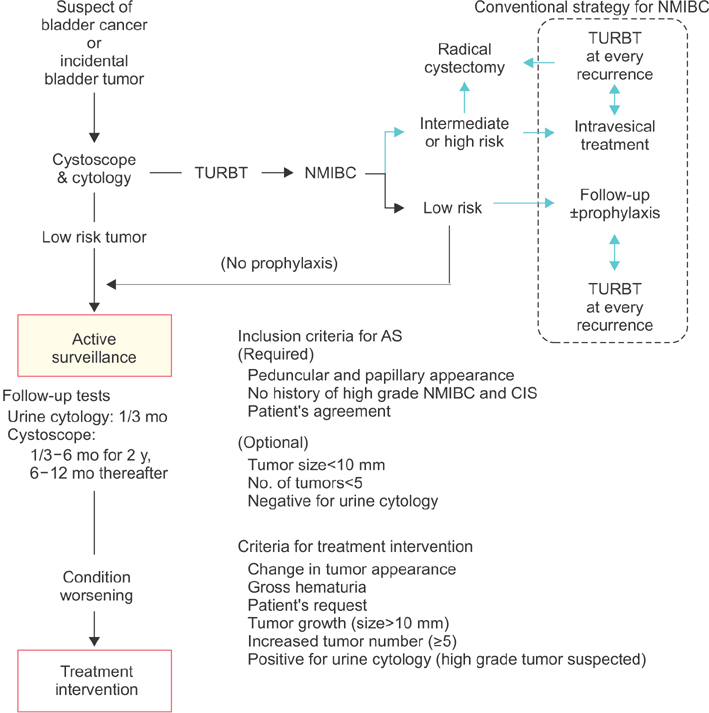
- Nonmuscle invasive bladder cancer (NMIBC) is known to be a heterogeneous malignancy that requires varying treatment modalities and follow-up schedules. Low-grade Ta papillary tumors are categorized as low-risk NMIBC because of their favorable prognosis. There is an expanding movement that overdiagnosis and overtreatment should be avoided considering the economic impact and the patients' quality of life. It has been over 10 years since the initial assessment of active surveillance for low-risk NMIBC suggested its feasibility and safety. However, urologists are still unfamiliar with this treatment option, which can be ideal in appropriately selected patients. In this review article, we focus on active surveillance for low-risk NMIBC and discuss the evidence and rationale for this treatment option. There are several issues to resolve in order to advocate active surveillance as a standard option in selected patients. A specific follow-up protocol including intervals of cystoscopy, urine cytology, urine markers, and other radiographic examinations need to be optimized and validated. Finally, we integrate the available data into the follow-up strategy and propose a new surveillance protocol for active surveillance of recurrent low-risk bladder cancer.
MeSH Terms
Figure
Cited by 1 articles
-
Suppression of CD81 promotes bladder cancer cell invasion through increased matrix metalloproteinase expression via extracellular signal-regulated kinase phosphorylation
Hyun Sik Park, Suhyuk Lee, Jisu Lee, Hyun Bin Shin, Seung-Min Yoo, Myung-Shin Lee, Jinsung Park
Investig Clin Urol. 2019;60(5):396-404. doi: 10.4111/icu.2019.60.5.396.
Reference
-
1. Bruinsma SM, Bangma CH, Carroll PR, Leapman MS, Rannikko A, Petrides N, et al. Active surveillance for prostate cancer: a narrative review of clinical guidelines. Nat Rev Urol. 2016; 13:151–167.2. Klotz L. Active surveillance and focal therapy for low-intermediate risk prostate cancer. Transl Androl Urol. 2015; 4:342–354.3. Bahouth Z, Halachmi S, Meyer G, Avitan O, Moskovitz B, Nativ O. The natural history and predictors for intervention in patients with small renal mass undergoing active surveillance. Adv Urol. 2015; 2015:692014.4. Ha SC, Zlomke HA, Cost N, Wilson S. The past, present, and future in management of small renal masses. J Oncol. 2015; 2015:364807.5. Heidenreich A. Clinical stage I nonseminomatous testicular germ-cell tumors: surgery or watchful waiting, still an issue? Curr Opin Urol. 2002; 12:427–430.6. Alhashemi A, Goldstein DP, Sawka AM. A systematic review of primary active surveillance management of low-risk papillary carcinoma. Curr Opin Oncol. 2016; 28:11–17.7. Soloway MS, Bruck DS, Kim SS. Expectant management of small, recurrent, noninvasive papillary bladder tumors. J Urol. 2003; 170(2 Pt 1):438–441.8. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.9. Editorial Board of the cancer Statistics in Japan. Cancer statistic in Japan 2013. Tokyo: Foundation for Promotion of Cancer Research;2013.10. Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013; 64:639–653.11. van Rhijn BW, Burger M, Lotan Y, Solsona E, Stief CG, Sylvester RJ, et al. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur Urol. 2009; 56:430–442.12. Miyake M, Gotoh D, Shimada K, Tatsumi Y, Nakai Y, Anai S, et al. Exploration of risk factors predicting outcomes for primary T1 high-grade bladder cancer and validation of the Spanish Urological Club for Oncological Treatment scoring model: long-term follow-up experience at a single institute. Int J Urol. 2015; 22:541–547.13. Sugano K, Kakizoe T. Genetic alterations in bladder cancer and their clinical applications in molecular tumor staging. Nat Clin Pract Urol. 2006; 3:642–652.14. Kikuchi E, Fujimoto H, Mizutani Y, Okajima E, Koga H, Hinotsu S, et al. Clinical outcome of tumor recurrence for Ta, T1 non-muscle invasive bladder cancer from the data on registered bladder cancer patients in Japan: 1999-2001 report from the Japanese Urological Association. Int J Urol. 2009; 16:279–286.15. Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006; 49:466–465.16. Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007; 178:2314–2330.17. Tiu A, Jenkins LC, Soloway MS. Active surveillance for low-risk bladder cancer. Urol Oncol. 2014; 32:33.e7–33.e10.18. Kobayashi H, Kikuchi E, Mikami S, Maeda T, Tanaka N, Miyajima A, et al. Long term follow-up in patients with initially diagnosed low grade Ta non-muscle invasive bladder tumors: tumor recurrence and worsening progression. BMC Urol. 2014; 14:5.19. Ozono S, Hinotsu S, Tabata S, Takashima K, Fujimoto K, Okajima E, et al. Treated natural history of superficial bladder cancer. Jpn J Clin Oncol. 2001; 31:536–540.20. Soloway MS, Sofer M, Vaidya A. Contemporary management of stage T1 transitional cell carcinoma of the bladder. J Urol. 2002; 167:1573–1583.21. Schwaibold HE, Sivalingam S, May F, Hartung R. The value of a second transurethral resection for T1 bladder cancer. BJU Int. 2006; 97:1199–1201.22. Herr HW. Does cystoscopy correlate with the histology of recurrent papillary tumours of the bladder? BJU Int. 2001; 88:683–685.23. Ito N. Early changes caused by N-butyl-N-(4-hydroxybutyl) nitrosamine in the bladder epithelium of different animal species. Cancer Res. 1976; 36(7 PT 2):2528–2531.24. Ohtani M, Kakizoe T, Sato S, Sugimura T, Fukushima S. Strain differences in mice with invasive bladder carcinomas induced by N-butyl-N-(4-hydroxybutyl)nitrosamine. J Cancer Res Clin Oncol. 1986; 112:107–110.25. Ito N, Hiasa Y, Tamai A, Okajima E, Kitamura H. Histogenesis of urinary bladder tumors induced by N-butyl-N-(4-hydroxybutyl) nitrosamine in rats. Gan. 1969; 60:401–410.26. Okajima E, Hiramatsu T, Motomiya Y, Kondo T, Hirao Y. Effects of foreign bodies on development of urinary bladder tumors in rats treated with N-butyl-N-(4-hydroxybutyl) nitrosamine. Urol Res. 1973; 1:177–181.27. Okajima E, Hiramatsu T, Iriya K, Ijuin M, Matsushima S. Effects of sex hormones on development of urinary bladder tumours in rats induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. Urol Res. 1975; 3:73–79.28. Okajima E, Hiramatsu T, Hirao K, Ijuin M, Hirao Y, Babaya K, et al. Urinary bladder tumors induced by N-butyl-N-(4-hydroxybutyl)nitrosamine in dogs. Cancer Res. 1981; 41:1958–1966.29. Tsunemi K, Ozono S, Yamaguchi H, Hayashi Y, Babaya K, Hirao Y, et al. Hisotpathological findings of the urinary bladder epithelium in aged dogs. J Toxicol Pathol. 1994; 7:73–80.30. Okajima E, Ozono S, Yoshida K, Samma S, Momose H, Iwai A, et al. A histopathological mapping study of the urinary bladder tumors induced by N-butyl-N-(4-hydroxybutyl)nitrosamine in dogs. Urol Res. 1997; 25:315–323.31. Collado A, Chechile GE, Salvador J, Vicente J. Early complications of endoscopic treatment for superficial bladder tumors. J Urol. 2000; 164:1529–1532.32. Nieder AM, Meinbach DS, Kim SS, Soloway MS. Transurethral bladder tumor resection: intraoperative and postoperative complications in a residency setting. J Urol. 2005; 174:2307–2309.33. Manikandan R, Lynch N, Grills RJ. Percutaneous peritoneal drainage for intraperitoneal bladder perforations during transurethral resection of bladder tumors. J Endourol. 2003; 17:945–947.34. Smith ZL, Soloway MS. Expectant management of low-risk bladder cancer. Curr Urol Rep. 2015; 16:82.35. Balbay MD, Cimentepe E, Unsal A, Bayrak O, Koc A, Akbulut Z. The actual incidence of bladder perforation following transurethral bladder surgery. J Urol. 2005; 174:2260–2262.36. Gofrit ON, Pode D, Lazar A, Katz R, Shapiro A. Watchful waiting policy in recurrent Ta G1 bladder tumors. Eur Urol. 2006; 49:303–306.37. Pruthi RS, Baldwin N, Bhalani V, Wallen EM. Conservative management of low risk superficial bladder tumors. J Urol. 2008; 179:87–90.38. Gofrit ON, Shapiro A. Active surveillance of low grade bladder tumors. Arch Ital Urol Androl. 2008; 80:132–135.39. Hernandez V, Alvarez M, de la Pena E, Amaruch N, Martín MD, de la Morena JM, et al. Safety of active surveillance program for recurrent nonmuscle-invasive bladder carcinoma. Urology. 2009; 73:1306–1310.40. Hernandez V, Llorente C, de la Pena E, Perez-Fernandez E, Guijarro A, Sola I. Long-term oncological outcomes of an active surveillance program in recurrent low grade Ta bladder cancer. Urol Oncol. 2016; 34:165.e19–165.e23.41. Oosterlinck W, Solsona E, Akaza H, Busch C, Goebell PJ, Malmstrom PU, et al. Low-grade Ta (noninvasive) urothelial carcinoma of the bladder. Urology. 2005; 66:6 Suppl 1. 75–89.42. Burger M, Grossman HB, Droller M, Schmidbauer J, Hermann G, Dragoescu O, et al. Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: a meta-analysis of detection and recurrence based on raw data. Eur Urol. 2013; 64:846–854.43. Altobelli E, Zlatev DV, Liao JC. Role of narrow band imaging in management of urothelial carcinoma. Curr Urol Rep. 2015; 16:58.44. Gupta M, Su LM. Current and evolving uses of optical coherence tomography in the genitourinary tract. Curr Urol Rep. 2015; 16:15.45. Chen SP, Liao JC. Confocal laser endomicroscopy of bladder and upper tract urothelial carcinoma: a new era of optical diagnosis? Curr Urol Rep. 2014; 15:437.46. Nakai Y, Anai S, Onishi S, Masaomi K, Tatsumi Y, Miyake M, et al. Protoporphyrin IX induced by 5-aminolevulinic acid in bladder cancer cells in voided urine can be extracorporeally quantified using a spectrophotometer. Photodiagnosis Photodyn Ther. 2015; 12:282–288.47. Hirao Y, Okajima E, Ozono S, Samma S, Sasaki K, Hiramatsu T, et al. A prospective randomized study of prophylaxis of tumor recurrence following transurethral resection of superficial bladder cancer: intravesical thio-TEPA versus oral UFT. Cancer Chemother Pharmacol. 1992; 30:Suppl. S26–S30.48. Bell MD, Yafi FA, Brimo F, Steinberg J, Aprikian AG, Tanguay S, et al. Prognostic value of urinary cytology and other biomarkers for recurrence and progression in bladder cancer: a prospective study. World J Urol. 2016; 02. 23. [Epub]. DOI: 10.1007/s00345-016-1795-5.49. Shariat SF, Zippe C, Ludecke G, Boman H, Sanchez-Carbayo M, Casella R, et al. Nomograms including nuclear matrix protein 22 for prediction of disease recurrence and progression in patients with Ta, T1 or CIS transitional cell carcinoma of the bladder. J Urol. 2005; 173:1518–1525.50. Habuchi T, Marberger M, Droller MJ, Hemstreet GP 3rd, Grossman HB, Schalken JA, et al. Prognostic markers for bladder cancer: International Consensus Panel on bladder tumor markers. Urology. 2005; 66:6 Suppl 1. 64–74.51. Kompier LC, van Tilborg AA, Zwarthoff EC. Bladder cancer: novel molecular characteristics, diagnostic, and therapeutic implications. Urol Oncol. 2010; 28:91–96.52. Isharwal S, Konety B. Non-muscle invasive bladder cancer risk stratification. Indian J Urol. 2015; 31:289–296.53. van Rhijn BW, Vis AN, van der Kwast TH, Kirkels WJ, Radvanyi F, Ooms EC, et al. Molecular grading of urothelial cell carcinoma with fibroblast growth factor receptor 3 and MIB-1 is superior to pathologic grade for the prediction of clinical outcome. J Clin Oncol. 2003; 21:1912–1921.54. Miyake M, Fujimoto K, Anai S, Ohnishi S, Nakai Y, Inoue T, et al. Clinical significance of heme oxygenase-1 expression in non-muscle-invasive bladder cancer. Urol Int. 2010; 85:355–363.55. Hernandez S, Lopez-Knowles E, Lloreta J, Kogevinas M, Amoros A, Tardon A, et al. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. J Clin Oncol. 2006; 24:3664–3671.56. Miyake M, Sugano K, Sugino H, Imai K, Matsumoto E, Maeda K, et al. Fibroblast growth factor receptor 3 mutation in voided urine is a useful diagnostic marker and significant indicator of tumor recurrence in non-muscle invasive bladder cancer. Cancer Sci. 2010; 101:250–258.57. Miyake M, Ishii M, Koyama N, Kawashima K, Kodama T, Anai S, et al. 1-tert-butyl-3-[6-(3,5-dimethoxy-phenyl)-2-(4-diethylamino-butylamino)-pyrido[2,3-d]pyrimidin-7-yl]-urea (PD173074), a selective tyrosine kinase inhibitor of fibroblast growth factor receptor-3 (FGFR3), inhibits cell proliferation of bladder cancer carrying the FGFR3 gene mutation along with up-regulation of p27/Kip1 and G1/G0 arrest. J Pharmacol Exp Ther. 2010; 332:795–802.58. Fujimoto K, Yamada Y, Okajima E, Kakizoe T, Sasaki H, Sugimura T, et al. Frequent association of p53 gene mutation in invasive bladder cancer. Cancer Res. 1992; 52:1393–1398.59. Malats N, Bustos A, Nascimento CM, Fernandez F, Rivas M, Puente D, et al. P53 as a prognostic marker for bladder cancer: a meta-analysis and review. Lancet Oncol. 2005; 6:678–686.60. Mian C, Lodde M, Comploj E, Lusuardi L, Palermo S, Mian M, et al. Multiprobe fluorescence in situ hybridisation: prognostic perspectives in superficial bladder cancer. J Clin Pathol. 2006; 59:984–987.61. Fujimoto K, Tanaka Y, Rademaker A, Oyasu R. Epidermal growth factor-responsive and -refractory carcinomas initiated with N-methyl-N-nitrosourea in rat urinary bladder. Cancer Res. 1996; 56:2666–2670.62. Miyake M, Nakai Y, Anai S, Tatsumi Y, Kuwada M, Onishi S, et al. Diagnostic approach for cancer cells in urine sediments by 5-aminolevulinic acid-based photodynamic detection in bladder cancer. Cancer Sci. 2014; 105:616–622.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Autophagy and urothelial carcinoma of the bladder: A review
- Recombinant Bacille Calmette–Guérin for Immunotherapy in Nonmuscle Invasive Bladder Cancer
- Treatment Case of Asymptomatic Prostate Tuberculosis That Developed After Bacillus Calmette-Guerin Intravesical Therapy in a Patient With Nonmuscle Invasive Bladder Cancer
- Utility of Urinalysis as a Follow-up Surveillance Tool in Nonmuscle Invasive Bladder Cancer
- New Drugs for Bacillus Calmette Guérin-Unresponsive Nonmuscle Invasive Bladder Cancer