Yonsei Med J.
2018 Dec;59(10):1222-1231. 10.3349/ymj.2018.59.10.1222.
German Cockroach Extract Induces Matrix Metalloproteinase-1 Expression, Leading to Tight Junction Disruption in Human Airway Epithelial Cells
- Affiliations
-
- 1Department of Pediatrics, Institute of Allergy, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. mhsohn@yuhs.ac
- 2Department of Pediatrics, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea.
- 3Sowha Children's Hospital, Seoul, Korea.
- KMID: 2426337
- DOI: http://doi.org/10.3349/ymj.2018.59.10.1222
Abstract
- PURPOSE
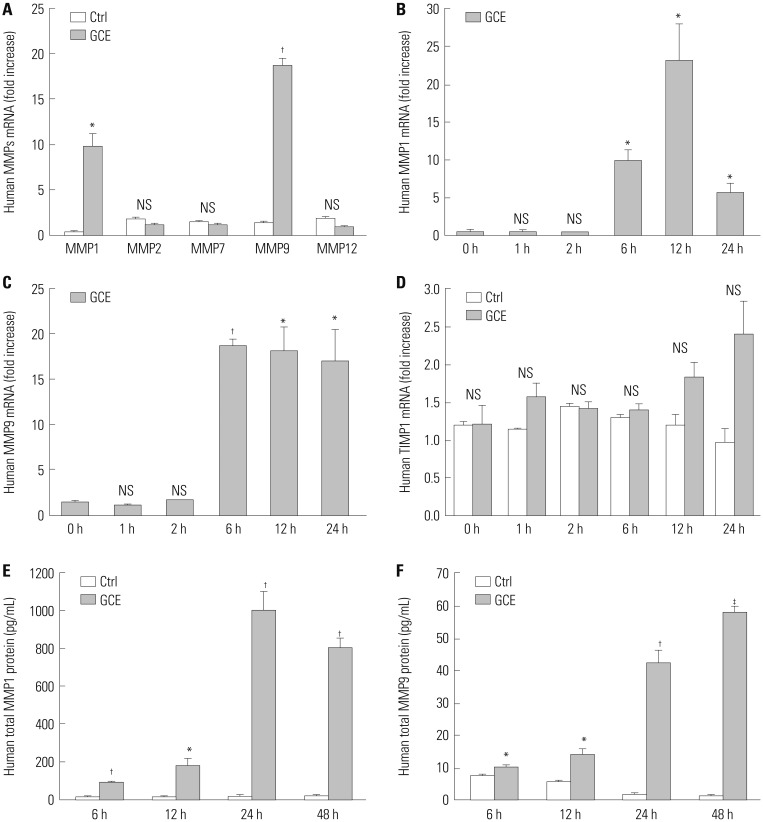
Cockroach exposure is a pivotal cause of asthma. Tight junctions are intercellular structures required for maintenance of the barrier function of the airway epithelium, which is impaired in this disease. Matrix metalloproteinases (MMPs) digest extracellular matrix components and are involved in asthma pathogenesis: MMP1 is a collagenase with a direct influence on airway obstruction in asthmatics. This study aimed to investigate the mechanism by which German cockroach extract (GCE) induces MMP1 expression and whether MMP1 release alters cellular tight junctions in human airway epithelial cells (NCI-H292).
MATERIALS AND METHODS
mRNA and protein levels were determined using real-time PCR and ELISA. Tight junction proteins were detected using immunofluorescence staining. Epithelial barrier function was measured by transepithelial electrical resistance (TEER). The binding of a transcription factor to DNA molecules was determined by electrophoretic mobility shift assay, while the levels of tight junction proteins and phosphorylation were determined using Western blotting.
RESULTS
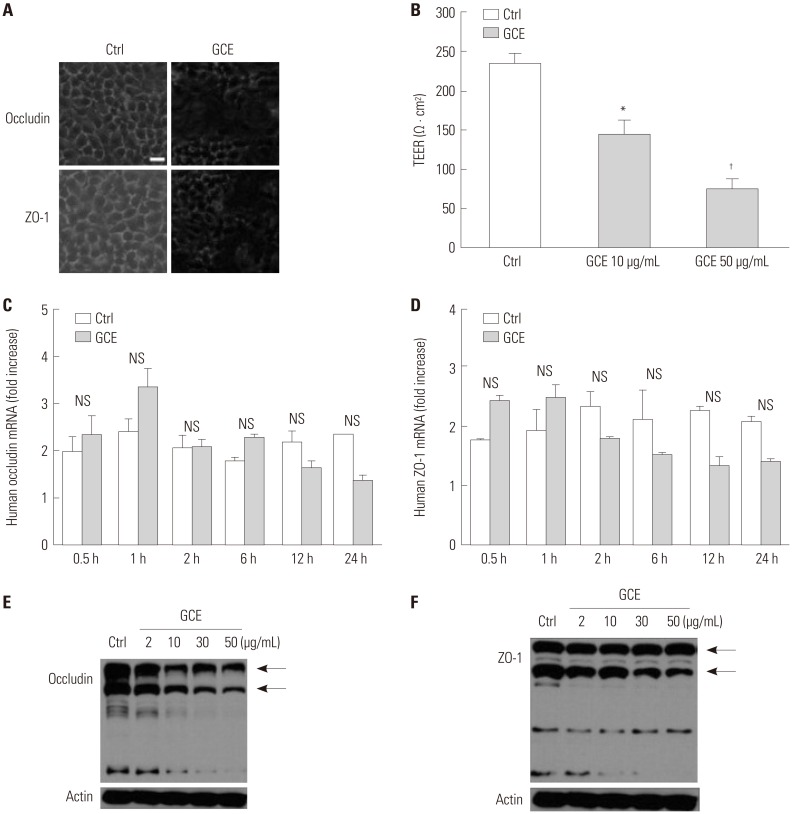
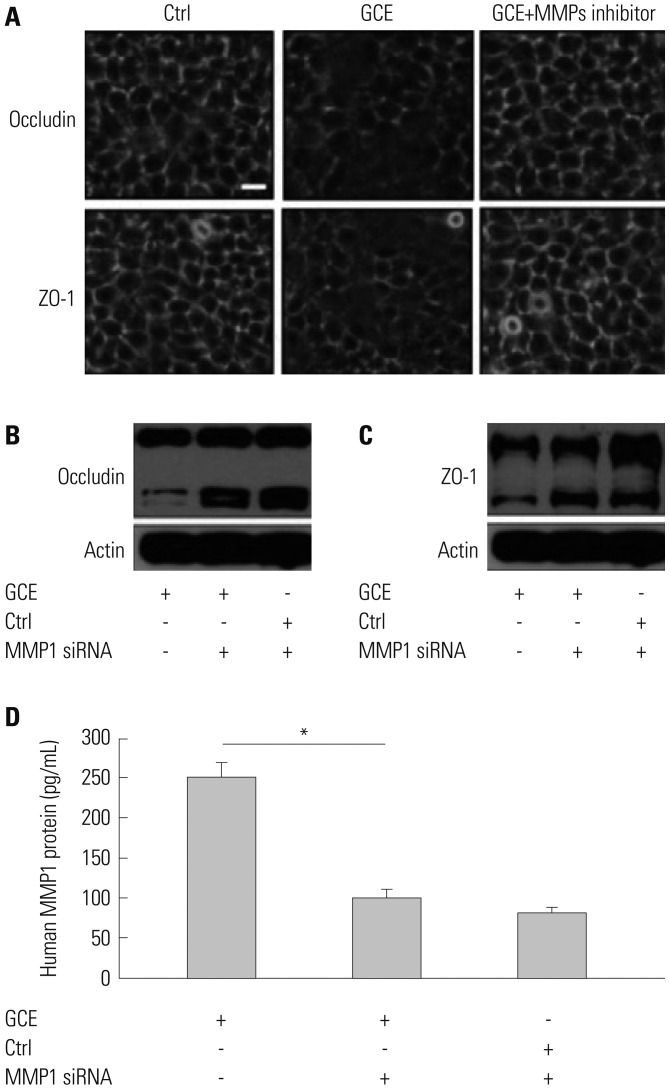
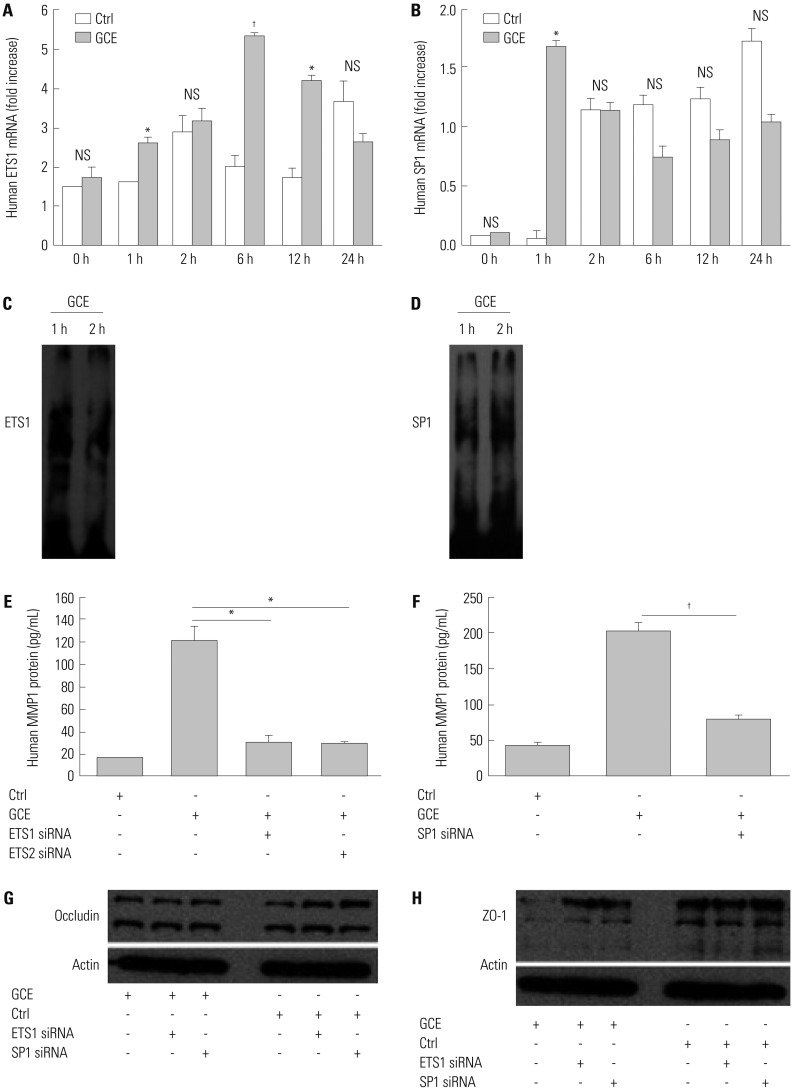
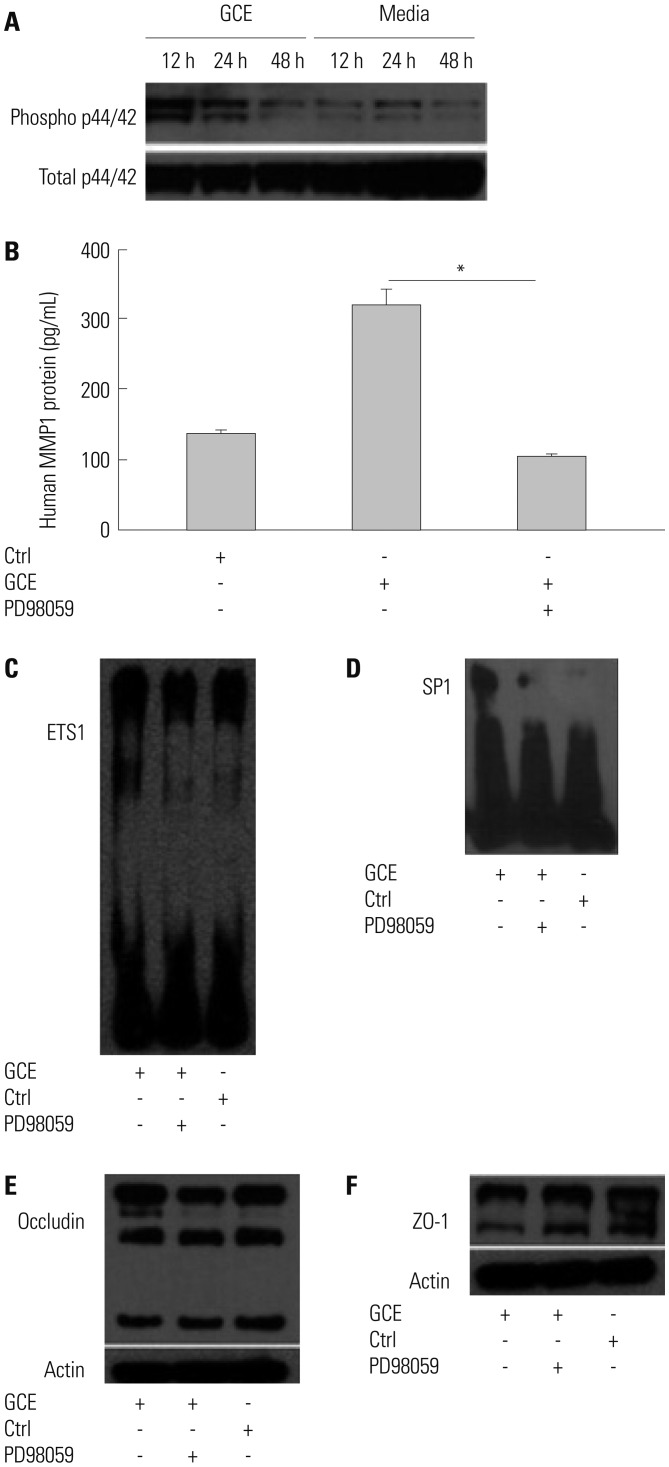
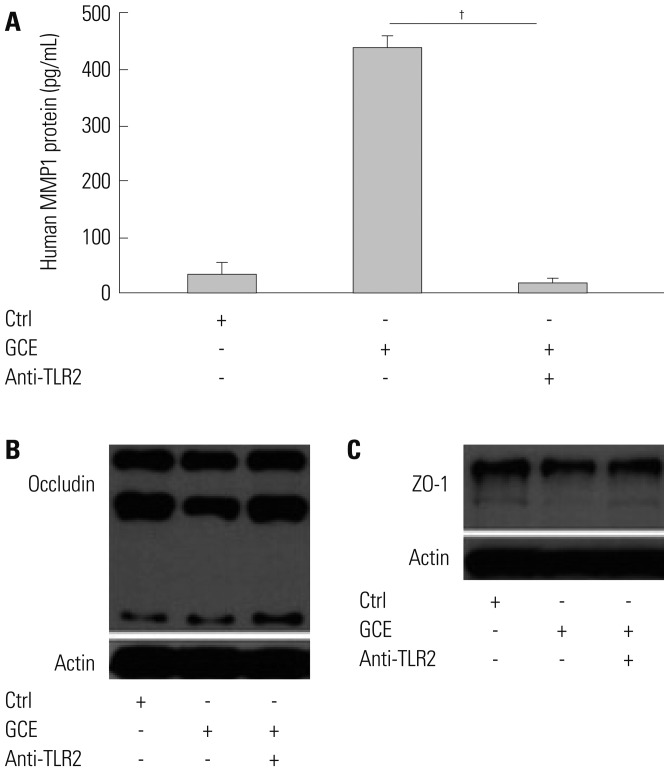
GCE was shown to increase MMP1 expression, TEER, and tight junction degradation. Both an inhibitor and small interfering RNA (siRNA) of MMP1 significantly decreased GCE-induced tight junction disruption. Furthermore, transient transfection with ETS1 and SP1 siRNA, and anti-TLR2 antibody pretreatment prevented MMP1 expression and tight junction degradation. An extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) inhibitor also blocked MMP1 release, ETS1/SP1 DNA binding, and tight junction alteration.
CONCLUSION
GCE treatment increases MMP1 expression, leading to tight junction disruption, which is transcriptionally regulated and influenced by the ERK/MAPK pathway in airway epithelial cells. These findings may contribute to developing novel therapeutic strategies for airway diseases.
MeSH Terms
-
Airway Obstruction
Asthma
Blattellidae*
Blotting, Western
Cockroaches
Collagenases
DNA
Electric Impedance
Electrophoretic Mobility Shift Assay
Enzyme-Linked Immunosorbent Assay
Epithelial Cells*
Epithelium
Extracellular Matrix
Fluorescent Antibody Technique
Humans*
Matrix Metalloproteinase 1*
Matrix Metalloproteinases
Phosphorylation
Phosphotransferases
Protein Kinases
Real-Time Polymerase Chain Reaction
RNA, Messenger
RNA, Small Interfering
Tight Junction Proteins
Tight Junctions*
Transcription Factors
Transfection
Collagenases
DNA
Matrix Metalloproteinase 1
Matrix Metalloproteinases
Phosphotransferases
Protein Kinases
RNA, Messenger
RNA, Small Interfering
Tight Junction Proteins
Transcription Factors
Figure
Reference
-
1. Lombardi C, Savi E, Ridolo E, Passalacqua G, Canonica GW. Is allergic sensitization relevant in severe asthma? Which allergens may be culprit? World Allergy Organ J. 2017; 10:2. PMID: 28101292.2. Sohn MH, Kim KE. The cockroach and allergic diseases. Allergy Asthma Immunol Res. 2012; 4:264–269. PMID: 22950031.
Article3. O'Hollaren MT, Yunginger JW, Offord KP, Somers MJ, O'Connell EJ, Ballard DJ, et al. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N Engl J Med. 1991; 324:359–363. PMID: 1987459.4. Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997; 336:1356–1363. PMID: 9134876.
Article5. Page K. Role of cockroach proteases in allergic disease. Curr Allergy Asthma Rep. 2012; 12:448–455. PMID: 22644866.
Article6. Yong TS, Lee JS, Lee J, Park SJ, Jeon SH, Ree HI, et al. Identification and purification of IgE-reactive proteins in German cockroach extract. Yonsei Med J. 1999; 40:283–289. PMID: 10412341.
Article7. Page K, Lierl KM, Hughes VS, Zhou P, Ledford JR, Wills-Karp M. TLR2-mediated activation of neutrophils in response to German cockroach frass. J Immunol. 2008; 180:6317–6324. PMID: 18424755.
Article8. Hughes VS, Page K. German cockroach frass proteases cleave pro-matrix metalloproteinase-9. Exp Lung Res. 2007; 33:135–150. PMID: 17558676.
Article9. Vandenbroucke RE, Dejonckheere E, Libert C. A therapeutic role for matrix metalloproteinase inhibitors in lung diseases? Eur Respir J. 2011; 38:1200–1214. PMID: 21659416.
Article10. Grzela K, Strzelak A, Zagorska W, Grzela T. Matrix metalloproteinases in asthma-associated airway remodeling-Dr. Jekyll or Mr. Hyde?. In : Pereira C, editor. Asthma-from childhood asthma to ACOS phenotypes. London: InTech;2016. p. 41–69.11. Fingleton B. MMPs as therapeutic targets--still a viable option? Semin Cell Dev Biol. 2008; 19:61–68. PMID: 17693104.
Article12. Suzuki R, Kato T, Miyazaki Y, Iwata M, Noda Y, Takagi K, et al. Matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in sputum from patients with bronchial asthma. J Asthma. 2001; 38:477–484. PMID: 11642414.13. Royce SG, Shen M, Patel KP, Huuskes BM, Ricardo SD, Samuel CS. Mesenchymal stem cells and serelaxin synergistically abrogate established airway fibrosis in an experimental model of chronic allergic airways disease. Stem Cell Res. 2015; 15:495–505. PMID: 26426509.
Article14. Holgate ST. A brief history of asthma and its mechanisms to modern concepts of disease pathogenesis. Allergy Asthma Immunol Res. 2010; 2:165–171. PMID: 20592914.
Article15. Loxham M, Davies DE, Blume C. Epithelial function and dysfunction in asthma. Clin Exp Allergy. 2014; 44:1299–1313. PMID: 24661647.
Article16. Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006; 22:207–235. PMID: 16771626.
Article17. Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol. 2007; 120:1233–1244. PMID: 18073119.
Article18. Holgate ST. The airway epithelium is central to the pathogenesis of asthma. Allergol Int. 2008; 57:1–10. PMID: 18209502.
Article19. Vermeer PD, Denker J, Estin M, Moninger TO, Keshavjee S, Karp P, et al. MMP9 modulates tight junction integrity and cell viability in human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2009; 296:L751–L762. PMID: 19270179.
Article20. Rogers NK, Clements D, Dongre A, Harrison TW, Shaw D, Johnson SR. Extra-cellular matrix proteins induce matrix metalloproteinase-1 (MMP-1) activity and increase airway smooth muscle contraction in asthma. PLoS One. 2014; 9:e90565. PMID: 24587395.
Article21. Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol. 2007; 211:19–26. PMID: 17167774.
Article22. Hadler-Olsen E, Fadnes B, Sylte I, Uhlin-Hansen L, Winberg JO. Regulation of matrix metalloproteinase activity in health and disease. FEBS J. 2011; 278:28–45. PMID: 21087458.
Article23. Antony AB, Tepper RS, Mohammed KA. Cockroach extract antigen increases bronchial airway epithelial permeability. J Allergy Clin Immunol. 2002; 110:589–595. PMID: 12373266.
Article24. Grzela K, Litwiniuk M, Zagorska W, Grzela T. Airway remodeling in chronic obstructive pulmonary disease and asthma: the role of matrix metalloproteinase-9. Arch Immunol Ther Exp (Warsz). 2016; 64:47–55.
Article25. Erlewyn-Lajeunesse MD, Hunt LP, Pohunek P, Dobson SJ, Kochhar P, Warner JA, et al. Bronchoalveolar lavage MMP-9 and TIMP-1 in preschool wheezers and their relationship to persistent wheeze. Pediatr Res. 2008; 64:194–199. PMID: 18391843.
Article26. Karakoc GB, Yukselen A, Yilmaz M, Altintas DU, Kendirli SG. Exhaled breath condensate MMP-9 level and its relationship with asthma severity and interleukin-4/10 levels in children. Ann Allergy Asthma Immunol. 2012; 108:300–304. PMID: 22541398.27. Rajah R, Nachajon RV, Collins MH, Hakonarson H, Grunstein MM, Cohen P. Elevated levels of the IGF-binding protein protease MMP-1 in asthmatic airway smooth muscle. Am J Respir Cell Mol Biol. 1999; 20:199–208. PMID: 9922210.
Article28. Dolhnikoff M, da Silva LF, de Araujo BB, Gomes HA, Fernezlian S, Mulder A, et al. The outer wall of small airways is a major site of remodeling in fatal asthma. J Allergy Clin Immunol. 2009; 123:1090–1097. PMID: 19361849.
Article29. Wan H, Winton HL, Soeller C, Gruenert DC, Thompson PJ, Cannell MB, et al. Quantitative structural and biochemical analyses of tight junction dynamics following exposure of epithelial cells to house dust mite allergen Der p 1. Clin Exp Allergy. 2000; 30:685–698. PMID: 10792361.
Article30. Blume C, Swindle EJ, Dennison P, Jayasekera NP, Dudley S, Monk P, et al. Barrier responses of human bronchial epithelial cells to grass pollen exposure. Eur Respir J. 2013; 42:87–97. PMID: 23143548.
Article31. Nhu QM, Shirey K, Teijaro JR, Farber DL, Netzel-Arnett S, Antalis TM, et al. Novel signaling interactions between proteinase-activated receptor 2 and Toll-like receptors in vitro and in vivo. Mucosal Immunol. 2010; 3:29–39. PMID: 19865078.
Article32. Rallabhandi P, Nhu QM, Toshchakov VY, Piao W, Medvedev AE, Hollenberg MD, et al. Analysis of proteinase-activated receptor 2 and TLR4 signal transduction: a novel paradigm for receptor cooperativity. J Biol Chem. 2008; 283:24314–24325. PMID: 18622013.33. Segura-Valdez L, Pardo A, Gaxiola M, Uhal BD, Becerril C, Selman M. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest. 2000; 117:684–694. PMID: 10712992.
Article34. Imai K, Dalal SS, Chen ES, Downey R, Schulman LL, Ginsburg M, et al. Human collagenase (matrix metalloproteinase-1) expression in the lungs of patients with emphysema. Am J Respir Crit Care Med. 2001; 163(3 Pt 1):786–791. PMID: 11254539.
Article35. Cataldo DD, Gueders M, Munaut C, Rocks N, Bartsch P, Foidart JM, et al. Matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases mRNA transcripts in the bronchial secretions of asthmatics. Lab Invest. 2004; 84:418–424. PMID: 14968124.
Article36. Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011; 128:549–556. PMID: 21752437.
Article37. Rutter JL, Mitchell TI, Butticè G, Meyers J, Gusella JF, Ozelius LJ, et al. A single nucleotide polymorphism in the matrix metallo-proteinase-1 promoter creates an Ets binding site and augments transcription. Cancer Res. 1998; 58:5321–5325. PMID: 9850057.38. Liu T, Wang P, Cong M, Zhang D, Liu L, Li H, et al. Matrix metalloproteinase-1 induction by diethyldithiocarbamate is regulated via Akt and ERK/miR222/ETS-1 pathways in hepatic stellate cells. Biosci Rep. 2016; 36:e00371. PMID: 27412967.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ampicillin treated German cockroach extract leads to reduced inflammation in human lung cells and a mouse model of Asthma
- PMA-induced up-regulation of MMP-9 is regulated by a PKCalpha-NF-kappaB cascade in human lung epithelial cells
- Effect of Epigallocatechin-3-Gallate on PMA-Induced MUC5B Expression in Human Airway Epithelial Cells
- Allergenic Characterization of a Novel Allergen, Homologous to Chymotrypsin, from German Cockroach
- IgE binding patterns to German cockroach whole body extract in Korean atopic asthmatic children