Yonsei Med J.
2018 Dec;59(10):1190-1196. 10.3349/ymj.2018.59.10.1190.
Ophthalmoplegia in Mitochondrial Disease
- Affiliations
-
- 1Department of Pediatrics, Gangnam Severance Hospital, Severance Children's Hospital, Yonsei University College of Medicine, Seoul, Korea. ymleemd@yuhs.ac
- 2Department of Ophthalmology, Institute of Vision Research, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 3Epilepsy Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2426333
- DOI: http://doi.org/10.3349/ymj.2018.59.10.1190
Abstract
- PURPOSE
To evaluate the classification, diagnosis, and natural course of ophthalmoplegia associated with mitochondrial disease.
MATERIALS AND METHODS
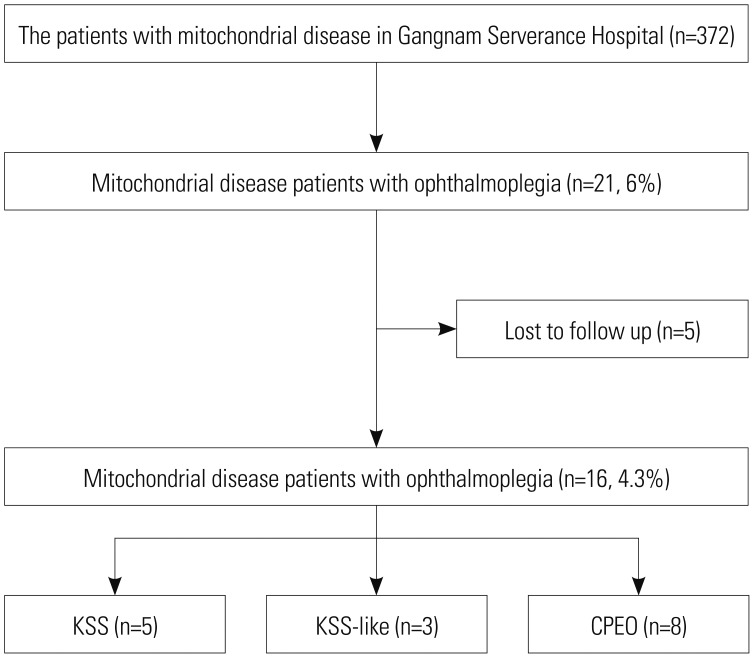
Among 372 patients with mitochondrial disease who visited our hospital between January 2006 and January 2016, 21 patients with ophthalmoplegia were retrospectively identified. Inclusion criteria included onset before 20 years of age, pigmentary retinopathy, and cardiac involvement. The 16 patients who were finally included in the study were divided into three groups according to disease type: Kearns-Sayre syndrome (KSS), KSS-like, and chronic progressive external ophthalmoplegia (CPEO).
RESULTS
The prevalences of clinical findings were as follows: ptosis and retinopathy, both over 80%; myopathy, including extraocular muscles, 75%; lactic acidosis, 71%; and elevated levels of serum creatine kinase, 47%. Half of the patients had normal magnetic resonance imaging findings. A biochemical enzyme assay revealed mitochondrial respiratory chain complex I defect as the most common (50%). The prevalence of abnormal muscle findings in light or electron microscopic examinations was 50% each, while that of large-scale mitochondrial DNA (mtDNA) deletions in a gene study was 25%. We compared the KSS and KSS-like groups with the CPEO patient group, which showed pigmentary retinopathy (p < 0.001), cardiac conduction disease (p=0.013), and large-scale mtDNA deletions (p=0.038). KSS and KSS-like groups also had gastrointestinal tract disorders such as abnormal gastrointestinal motility (p=0.013) unlike the CPEO group.
CONCLUSION
Patients with KSS had gastrointestinal symptoms, which may indicate another aspect of systemic involvement. The presence of large-scale mtDNA deletions was an objective diagnostic factor for KSS and a gene study may be helpful for evaluating patients with KSS.
Keyword
MeSH Terms
-
Acidosis, Lactic
Classification
Creatine Kinase
Diagnosis
DNA, Mitochondrial
Electron Transport
Enzyme Assays
Gastrointestinal Motility
Gastrointestinal Tract
Genes, vif
Humans
Kearns-Sayre Syndrome
Magnetic Resonance Imaging
Mitochondrial Diseases*
Muscles
Muscular Diseases
Ophthalmoplegia*
Ophthalmoplegia, Chronic Progressive External
Prevalence
Retinitis Pigmentosa
Retrospective Studies
Creatine Kinase
DNA, Mitochondrial
Figure
Cited by 2 articles
-
Mitochondrial Ophthalmoplegia Is Not Only due to mtDNA Deletions
Josef Finsterer
Yonsei Med J. 2019;60(2):230-231. doi: 10.3349/ymj.2019.60.2.230.The Author Reply: Genetic Data Are a Prerequisite for Interpreting Clinical and Muscle Biopsy Findings in MELAS
Young-Mock Lee
Yonsei Med J. 2019;60(4):401-401. doi: 10.3349/ymj.2019.60.4.401.
Reference
-
1. Eom S, Lee HN, Lee S, Kang HC, Lee JS, Kim HD, et al. Cause of death in children with mitochondrial diseases. Pediatr Neurol. 2017; 66:82–88. PMID: 27843091.
Article2. Yatsuga S, Povalko N, Nishioka J, Katayama K, Kakimoto N, Matsuishi T, et al. Taro Matsuoka for MELAS Study Group in Japan. MELAS: a nationwide prospective cohort study of 96 patients in Japan. Biochim Biophys Acta. 2012; 1820:619–624. PMID: 21443929.
Article3. Eom S, Lee YM. Preliminary study of neurodevelopmental outcomes and parenting stress in pediatric mitochondrial disease. Pediatr Neurol. 2017; 71:43–49. PMID: 28476522.
Article4. Haas RH, Parikh S, Falk MJ, Saneto RP, Wolf NI, Darin N, et al. Mitochondrial disease: a practical approach for primary care physicians. Pediatrics. 2007; 120:1326–1333. PMID: 18055683.
Article6. López-Gallardo E, López-Pérez MJ, Montoya J, Ruiz-Pesini E. CPEO and KSS differ in the percentage and location of the mtDNA deletion. Mitochondrion. 2009; 9:314–317. PMID: 19410662.
Article7. Heidenreich JO, Klopstock T, Schirmer T, Saemann P, Mueller-Felber W, Auer DP. Chronic progressive external ophthalmoplegia: MR spectroscopy and MR diffusion studies in the brain. AJR Am J Roentgenol. 2006; 187:820–824. PMID: 16928952.
Article8. Bernier FP, Boneh A, Dennett X, Chow CW, Cleary MA, Thorburn DR. Diagnostic criteria for respiratory chain disorders in adults and children. Neurology. 2002; 59:1406–1411. PMID: 12427892.9. Joyce NC, Oskarsson B, Jin LW. Muscle biopsy evaluation in neuromuscular disorders. Phys Med Rehabil Clin N Am. 2012; 23:609–631. PMID: 22938878.
Article10. Skladal D, Sudmeier C, Konstantopoulou V, Stöckler-Ipsiroglu S, Plecko-Startinig B, Bernert G, et al. The clinical spectrum of mitochondrial disease in 75 pediatric patients. Clin Pediatr (Phila). 2003; 42:703–710. PMID: 14601919.
Article11. Munnich A, Rötig A, Chretien D, Cormier V, Bourgeron T, Bonnefont JP, et al. Clinical presentation of mitochondrial disorders in childhood. J Inherit Metab Dis. 1996; 19:521–527. PMID: 8884575.12. DiMauro S, Schon EA. Mitochondrial respiratory-chain diseases. N Engl J Med. 2003; 348:2656–2668. PMID: 12826641.
Article13. Grönlund MA, Honarvar AK, Andersson S, Moslemi AR, Oldfors A, Holme E, et al. Ophthalmological findings in children and young adults with genetically verified mitochondrial disease. Br J Ophthalmol. 2010; 94:121–127. PMID: 20385529.14. Han J, Lee YM, Kim SM, Han SY, Lee JB, Han SH. Ophthalmological manifestations in patients with Leigh syndrome. Br J Ophthalmol. 2015; 99:528–535. PMID: 25351680.
Article15. Park SB, Ma KT, Kook KH, Lee SY. Kearns-Sayre syndrome: 3 case reports and review of clinical feature. Yonsei Med J. 2004; 45:727–735. PMID: 15344217.
Article16. Mkaouar-Rebai E, Chamkha I, Kammoun T, Chabchoub I, Aloulou H, Fendri N, et al. A case of Kearns-Sayre syndrome with two novel deletions (9.768 and 7.253 kb) of the mtDNA associated with the common deletion in blood leukocytes, buccal mucosa and hair follicles. Mitochondrion. 2010; 10:449–455. PMID: 20388556.17. Young TJ, Shah AK, Lee MH, Hayes DL. Kearns-Sayre syndrome: a case report and review of cardiovascular complications. Pacing Clin Electrophysiol. 2005; 28:454–457. PMID: 15869681.
Article18. García-Cazorla A, De Lonlay P, Nassogne MC, Rustin P, Touati G, Saudubray JM. Long-term follow-up of neonatal mitochondrial cytopathies: a study of 57 patients. Pediatrics. 2005; 116:1170–1177. PMID: 16264005.19. Debray FG, Lambert M, Chevalier I, Robitaille Y, Decarie JC, Shoubridge EA, et al. Long-term outcome and clinical spectrum of 73 pediatric patients with mitochondrial diseases. Pediatrics. 2007; 119:722–733. PMID: 17403843.
Article20. Scaglia F, Towbin JA, Craigen WJ, Belmont JW, Smith EO, Neish SR, et al. Clinical spectrum, morbidity, and mortality in 113 pediatric patients with mitochondrial disease. Pediatrics. 2004; 114:925–931. PMID: 15466086.
Article21. Darin N, Oldfors A, Moslemi AR, Holme E, Tulinius M. The incidence of mitochondrial encephalomyopathies in childhood: clinical features and morphological, biochemical, and DNA abnormalities. Ann Neurol. 2001; 49:377–383. PMID: 11261513.
Article22. Chawla S, Coku J, Forbes T, Kannan S. Kearns-Sayre syndrome presenting as complete heart block. Pediatr Cardiol. 2008; 29:659–662. PMID: 17763890.
Article23. Sharma AK, Jain N, Kharwar RB, Narain VS. Classical triad of Kearns-Sayre syndrome. BMJ Case Rep. 2016; 2016:bcr2016216500.
Article24. Kisler JE, Whittaker RG, McFarland R. Mitochondrial diseases in childhood: a clinical approach to investigation and management. Dev Med Child Neurol. 2010; 52:422–433. PMID: 20163433.
Article25. Choi HS, Lee YM. Enteral tube feeding in paediatric mitochondrial diseases. Sci Rep. 2017; 7:16909. PMID: 29203845.
Article26. Finsterer J. Overview on visceral manifestations of mitochondrial disorders. Neth J Med. 2006; 64:61–71. PMID: 16547358.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mitochondrial Ophthalmoplegia Is Not Only due to mtDNA Deletions
- The Author Reply: Mitochondrial Ophthalmoplegia Is Not Only due to mtDNA Deletions
- Mitochondrial Intestinal Pseudo-Obstruction with Neurogenic Bladder Syndrome: Point Mutation at T8356C: A New Mitochondrial Disease?
- Genetics of Mitochondrial Myopathies
- A Case of Kearns-Sayre Syudrome