Korean J Radiol.
2018 Dec;19(6):1053-1065. 10.3348/kjr.2018.19.6.1053.
Prediction of Local Tumor Progression after Radiofrequency Ablation (RFA) of Hepatocellular Carcinoma by Assessment of Ablative Margin Using Pre-RFA MRI and Post-RFA CT Registration
- Affiliations
-
- 1Department of Radiology, Seoul National University Hospital, Seoul 03080, Korea. jmsh@snu.ac.kr
- 2Department of Radiology, Seoul National University College of Medicine, Seoul 03080, Korea.
- 3Institute of Radiation Medicine, Seoul National University Medical Research Center, Seoul 03087, Korea.
- 4Siemens Healthcare, Forchheim 91301, Germany.
- 5Department of Radiology, SMG-SNU Boramae Medical Center, Seoul 07061, Korea.
- 6Department of Radiology, KonKuk University Medical Center, Seoul 05030, Korea.
- 7Department of Radiology, Chung-Ang University Hospital, Seoul 06973, Korea.
- KMID: 2424845
- DOI: http://doi.org/10.3348/kjr.2018.19.6.1053
Abstract
OBJECTIVE
To evaluate the clinical impact of using registration software for ablative margin assessment on pre-radiofrequency ablation (RFA) magnetic resonance imaging (MRI) and post-RFA computed tomography (CT) compared with the conventional side-by-side MR-CT visual comparison.
MATERIALS AND METHODS
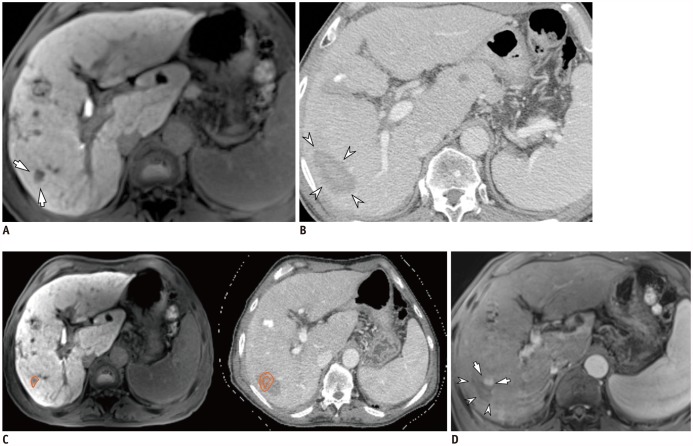
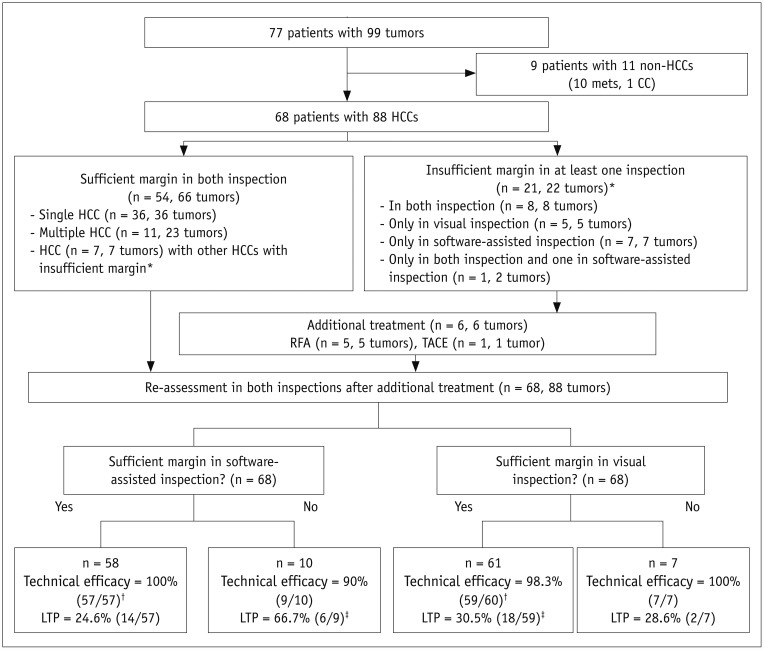
In this Institutional Review Board-approved prospective study, 68 patients with 88 hepatocellulcar carcinomas (HCCs) who had undergone pre-RFA MRI were enrolled. Informed consent was obtained from all patients. Pre-RFA MRI and post-RFA CT images were analyzed to evaluate the presence of a sufficient safety margin (≥ 3 mm) in two separate sessions using either side-by-side visual comparison or non-rigid registration software. Patients with an insufficient ablative margin on either one or both methods underwent additional treatment depending on the technical feasibility and patient's condition. Then, ablative margins were re-assessed using both methods. Local tumor progression (LTP) rates were compared between the sufficient and insufficient margin groups in each method.
RESULTS
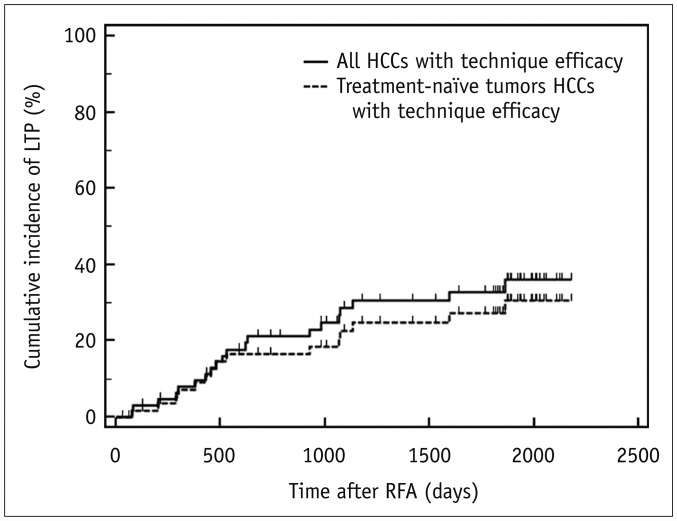
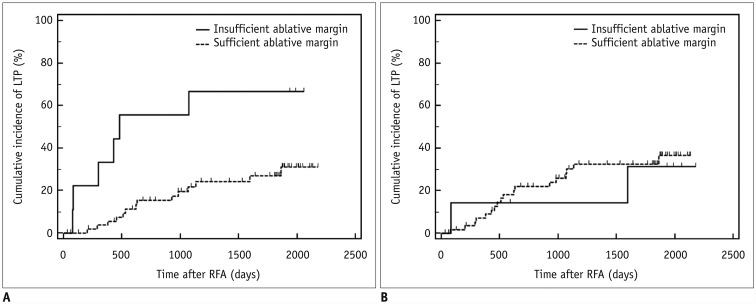
The two methods showed 14.8% (13/88) discordance in estimating sufficient ablative margins. On registration software-assisted inspection, patients with insufficient ablative margins showed a significantly higher 5-year LTP rate than those with sufficient ablative margins (66.7% vs. 27.0%, p = 0.004). However, classification by visual inspection alone did not reveal a significant difference in 5-year LTP between the two groups (28.6% vs. 30.5%, p = 0.79).
CONCLUSION
Registration software provided better ablative margin assessment than did visual inspection in patients with HCCs who had undergone pre-RFA MRI and post-RFA CT for prediction of LTP after RFA and may provide more precise risk stratification of those who are treated with RFA.
Keyword
MeSH Terms
Figure
Reference
-
1. Livraghi T, Solbiati L. [Percutaneous treatment: radiofrequency ablation of hepatic metastases in colorectal cancer]. Tumori. 2001; 87(1 Suppl 1):S69.2. Taner T, Atwell TD, Zhang L, Oberg TN, Harmsen WS, Slettedahl SW, et al. Adjunctive radiofrequency ablation of metastatic neuroendocrine cancer to the liver complements surgical resection. HPB (Oxford). 2013; 15:190–195. PMID: 23374359.
Article3. Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013; 59:300–307. PMID: 23603669.
Article4. Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012; 56:412–418. PMID: 21756858.
Article5. Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005; 234:961–967. PMID: 15665226.
Article6. Nakazawa T, Kokubu S, Shibuya A, Ono K, Watanabe M, Hidaka H, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007; 188:480–488. PMID: 17242258.
Article7. Wang X, Sofocleous CT, Erinjeri JP, Petre EN, Gonen M, Do KG, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013; 36:166–175. PMID: 22535243.
Article8. Sotirchos VS, Petrovic LM, Gönen M, Klimstra DS, Do RK, Petre EN, et al. Colorectal cancer liver metastases: biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology. 2016; 280:949–959. PMID: 27010254.
Article9. Shady W, Petre EN, Gonen M, Erinjeri JP, Brown KT, Covey AM, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes--A 10-year experience at a single center. Radiology. 2016; 278:601–611. PMID: 26267832.
Article10. Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010; 195:758–765. PMID: 20729457.11. Shin S, Lee JM, Kim KW, Joo I, Han JK, Choi BI, et al. Postablation assessment using follow-up registration of CT images before and after radiofrequency ablation (RFA): prospective evaluation of midterm therapeutic results of RFA for hepatocellular carcinoma. AJR Am J Roentgenol. 2014; 203:70–77. PMID: 24951197.
Article12. Kodama H, Yamakado K, Hasegawa T, Fujimori M, Yamanaka T, Takaki H, et al. Radiofrequency ablation using a multiple-Electrode switching system for lung tumors with 2.0–5.0-cm maximum diameter: phase II clinical study. Radiology. 2015; 277:895–902. PMID: 26053308.
Article13. Yoon JH, Lee JM, Woo S, Hwang EJ, Hwang I, Choi W, et al. Switching bipolar hepatic radiofrequency ablation using internally cooled wet electrodes: comparison with consecutive monopolar and switching monopolar modes. Br J Radiol. 2015; 88:20140468. PMID: 25873479.
Article14. Chinnaratha MA, Chuang MY, Fraser RJ, Woodman RJ, Wigg AJ. Percutaneous thermal ablation for primary hepatocellular carcinoma: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016; 31:294–301. PMID: 26114968.
Article15. Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. International Working Group on Image-Guided Tumor Ablation. Interventional Oncology Sans Frontières Expert Panel. Technology Assessment Committee of the Society of Interventional Radiology. Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. J Vasc Interv Radiol. 2014; 25:1691–1705.e4. PMID: 25442132.
Article16. Kim KW, Lee JM, Klotz E, Kim SJ, Kim SH, Kim JY, et al. Safety margin assessment after radiofrequency ablation of the liver using registration of preprocedure and postprocedure CT images. AJR Am J Roentgenol. 2011; 196:W565–W572. PMID: 21512046.
Article17. Gollub MJ, Hong R, Sarasohn DM, Akhurst T. Limitations of CT during PET/CT. J Nucl Med. 2007; 48:1583–1591. PMID: 17873133.
Article18. Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology. 2015; 275:97–109. PMID: 25559230.
Article19. Kierans AS, Kang SK, Rosenkrantz AB. The diagnostic performance of dynamic contrast-enhanced MR imaging for detection of small hepatocellular carcinoma measuring up to 2 cm: a meta-analysis. Radiology. 2016; 278:82–94. PMID: 26098460.
Article20. Elhawary H, Oguro S, Tuncali K, Morrison PR, Tatli S, Shyn PB, et al. Multimodality non-rigid image registration for planning, targeting and monitoring during CT-guided percutaneous liver tumor cryoablation. Acad Radiol. 2010; 17:1334–1344. PMID: 20817574.21. Archip N, Tatli S, Morrison P, Jolesz F, Warfield SK, Silverman S. Non-rigid registration of pre-procedural MR images with intra-procedural unenhanced CT images for improved targeting of tumors during liver radiofrequency ablations. Med Image Comput Comput Assist Interv. 2007; 10(Pt 2):969–977. PMID: 18044662.
Article22. Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208–1236. PMID: 16250051.
Article23. Hope TA, Fowler KJ, Sirlin CB, Costa EA, Yee J, Yeh BM, et al. Hepatobiliary agents and their role in LI-RADS. Abdom Imaging. 2015; 40:613–625. PMID: 25287679.
Article24. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30:52–60. PMID: 20175033.
Article25. Kim JW, Shin SS, Heo SH, Hong JH, Lim HS, Seon HJ, et al. Ultrasound-guided percutaneous radiofrequency ablation of liver tumors: how we do it safely and completely. Korean J Radiol. 2015; 16:1226–1239. PMID: 26576111.
Article26. Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. International Working Group on Image-guided Tumor Ablation. Interventional Oncology Sans Frontières Expert Panel. Technology Assessment Committee of the Society of Interventional Radiology. Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014; 273:241–260. PMID: 24927329.
Article27. Kang TW, Lim HK, Lee MW, Kim YS, Rhim H, Lee WJ, et al. Long-term therapeutic outcomes of radiofrequency ablation for subcapsular versus nonsubcapsular hepatocellular carcinoma: a propensity score matched study. Radiology. 2016; 280:300–312. PMID: 26824711.
Article28. Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977; 33:363–374. PMID: 884196.
Article29. Heizmann O, Zidowitz S, Bourquain H, Potthast S, Peitgen HO, Oertli D, et al. Assessment of intraoperative liver deformation during hepatic resection: prospective clinical study. World J Surg. 2010; 34:1887–1893. PMID: 20372896.
Article30. Crum WR, Hartkens T, Hill DL. Non-rigid image registration: theory and practice. Br J Radiol. 2004; 77(Spec No 2):S140–S153. PMID: 15677356.
Article31. Vasquez Osorio EM, Hoogeman MS, Mendez Romero A, Wielopolski P, Zolnay A, Heijmen BJ. Accurate CT/MR vessel-guided nonrigid registration of largely deformed livers. Med Phys. 2012; 39:2463–2477. PMID: 22559617.32. Kim YS, Rhim H, Lim HK, Choi D, Lee MW, Park MJ. Coagulation necrosis induced by radiofrequency ablation in the liver: histopathologic and radiologic review of usual to extremely rare changes. Radiographics. 2011; 31:377–390. PMID: 21415185.
Article33. Kirilova A, Lockwood G, Choi P, Bana N, Haider MA, Brock KK, et al. Three-dimensional motion of liver tumors using cine-magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2008; 71:1189–1195. PMID: 18258378.
Article34. Rosu M, Dawson LA, Balter JM, McShan DL, Lawrence TS, Ten Haken RK. Alterations in normal liver doses due to organ motion. Int J Radiat Oncol Biol Phys. 2003; 57:1472–1479. PMID: 14630287.
Article35. Liao M, Zhong X, Zhang J, Liu Y, Zhu Z, Wu H, et al. Radiofrequency ablation using a 10-mm target margin for small hepatocellular carcinoma in patients with liver cirrhosis: a prospective randomized trial. J Surg Oncol. 2017; 115:971–979. PMID: 28334430.
Article36. Lee DH, Lee JM, Lee JY, Kim SH, Han JK, Choi BI. Radiofrequency ablation for intrahepatic recurrent hepatocellular carcinoma: long-term results and prognostic factors in 168 patients with cirrhosis. Cardiovasc Intervent Radiol. 2014; 37:705–715. PMID: 23912493.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Combination of Transarterial Chemoebolization and Radiofrequency Ablation for Hepatocellular Carcinoma Treatment
- Recent technical advances in radiofrequency ablations for hepatocellular carcinoma
- Radiofrequency Ablation of Hepatocellular Carcinoma: Pros and Cons
- Radiofrequency Ablation for Hepatocellular Carcinoma
- Aggressive tumor recurrence after radiofrequency ablation for hepatocellular carcinoma