Anat Cell Biol.
2015 Sep;48(3):188-194. 10.5115/acb.2015.48.3.188.
Nfic regulates tooth root patterning and growth
- Affiliations
-
- 1Cluster for Craniofacial Development and Regeneration Research, Institute of Oral Biosciences, School of Dentistry, Chonbuk National University, Jeonju, Korea. oasis@jbnu.ac.kr
- 2Department of Oral Histology-Developmental Biology, Dental Research Institute, School of Dentistry, Seoul National University, Seoul, Korea.
- KMID: 2424821
- DOI: http://doi.org/10.5115/acb.2015.48.3.188
Abstract
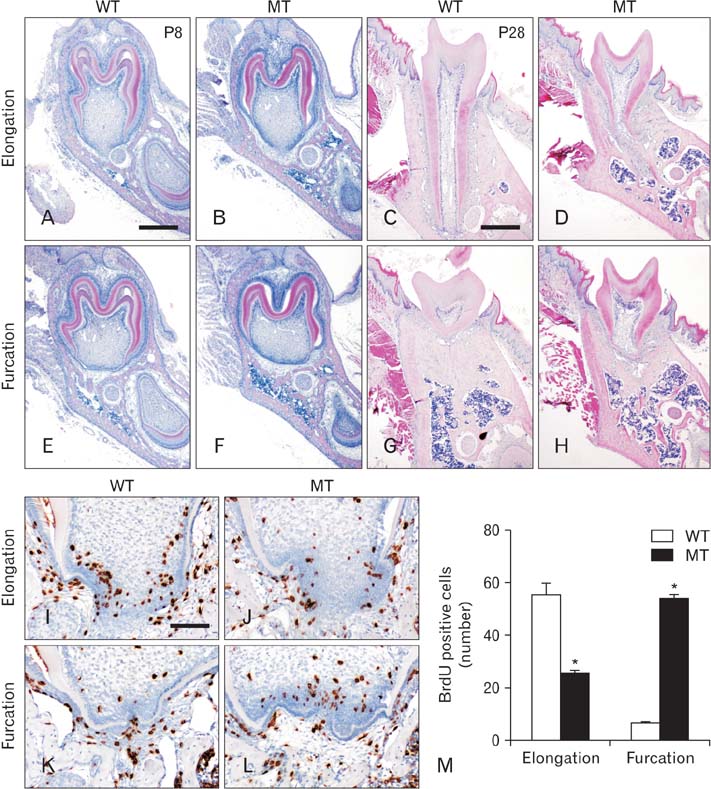
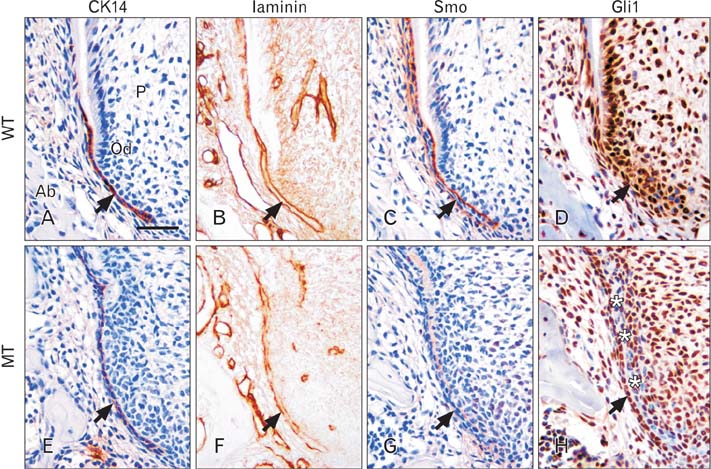
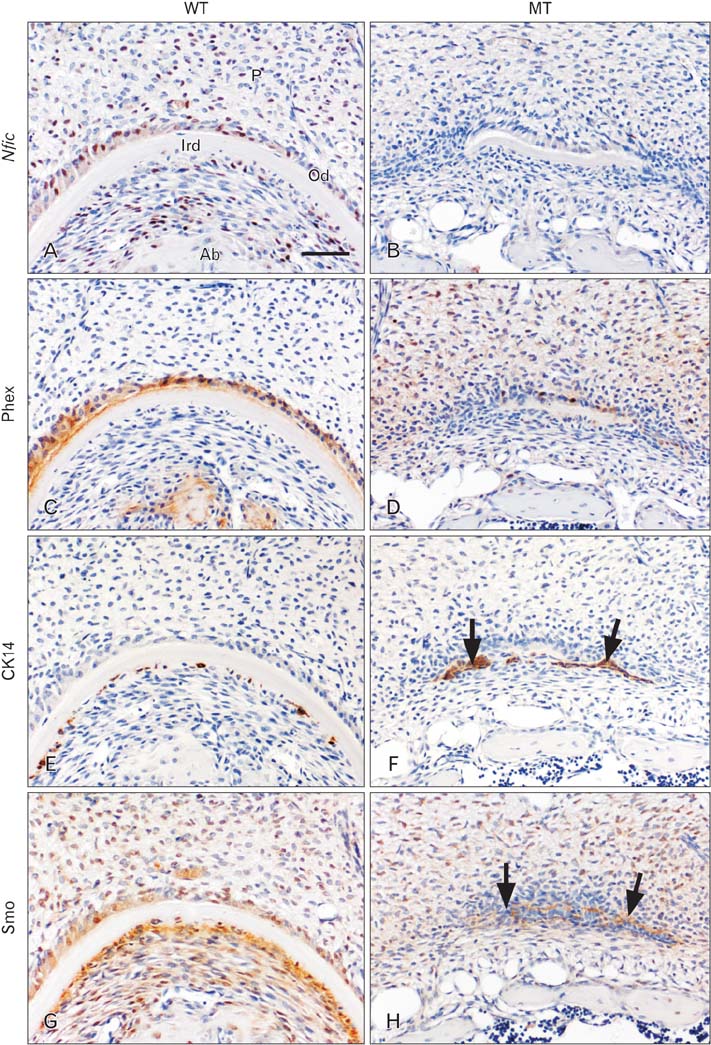
- Molecular interactions between epithelium and mesenchyme are important for root formation. Nuclear factor I-C (Nfic) has been identified as a key regulator of root formation. However, the mechanisms of root formation and their interactions between Hertwig's epithelial root sheath (HERS) and mesenchyme remain unclear. In this study, we investigated the role of Nfic in root patterning and growth during molar root development. The molars of Nfic knockout mice exhibited an enlarged pulp chamber and apical displacement of the pulpal floor, characteristic features of taurodontism, due to delayed furcation formation. In developing molar roots of mutant mice at P14, BrdU positive cells decreased in the apical mesenchyme of the elongation region whereas those cells increased in the dental papilla of the furcation region. Whereas cytokeratin 14 and laminin were localized in HERS cells of mutant molars, Smoothened (Smo) and Gli1 were downregulated in preodontoblasts. In contrast, cytokeratin 14 and Smo were localized in the cells of the furcation region of mutant molars. These results indicate that Nfic regulates cell proliferation in the dental mesenchyme and affects the fate of HERS cells in a site-specific manner. From the results, it is suggested that Nfic is required for root patterning and growth during root morphogenesis.
Keyword
MeSH Terms
Figure
Reference
-
1. Tummers M, Thesleff I. The importance of signal pathway modulation in all aspects of tooth development. J Exp Zool B Mol Dev Evol. 2009; 312B:309–319.2. Foster BL, Nociti FH Jr, Somerman MJ. Tooth root development. In : Huang GT, Thesleff I, editors. Stem Cells in Craniofacial Development and Regeneration. Hoboken: Wiley-Blackwell;2013. p. 153–177.3. Nakatomi M, Morita I, Eto K, Ota MS. Sonic hedgehog signaling is important in tooth root development. J Dent Res. 2006; 85:427–431.4. Huang X, Xu X, Bringas P Jr, Hung YP, Chai Y. Smad4-Shh-Nfic signaling cascade-mediated epithelial-mesenchymal interaction is crucial in regulating tooth root development. J Bone Miner Res. 2010; 25:1167–1178.5. Kim TH, Bae CH, Lee JC, Ko SO, Yang X, Jiang R, Cho ES. Beta-catenin is required in odontoblasts for tooth root formation. J Dent Res. 2013; 92:215–221.6. Gritli-Linde A, Bei M, Maas R, Zhang XM, Linde A, McMahon AP. Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development. 2002; 129:5323–5337.7. Gao Y, Yang G, Weng T, Du J, Wang X, Zhou J, Wang S, Yang X. Disruption of Smad4 in odontoblasts causes multiple keratocystic odontogenic tumors and tooth malformation in mice. Mol Cell Biol. 2009; 29:5941–5951.8. Steele-Perkins G, Butz KG, Lyons GE, Zeichner-David M, Kim HJ, Cho MI, Gronostajski RM. Essential role for NFI-C/CTF transcription-replication factor in tooth root development. Mol Cell Biol. 2003; 23:1075–1084.9. Park JC, Herr Y, Kim HJ, Gronostajski RM, Cho MI. Nfic gene disruption inhibits differentiation of odontoblasts responsible for root formation and results in formation of short and abnormal roots in mice. J Periodontol. 2007; 78:1795–1802.10. Lee DS, Park JT, Kim HM, Ko JS, Son HH, Gronostajski RM, Cho MI, Choung PH, Park JC. Nuclear factor I-C is essential for odontogenic cell proliferation and odontoblast differentiation during tooth root development. J Biol Chem. 2009; 284:17293–17303.11. Thomas HF. Root formation. Int J Dev Biol. 1995; 39:231–237.12. Sohn WJ, Choi MA, Yamamoto H, Lee S, Lee Y, Jung JK, Jin MU, An CH, Jung HS, Suh JY, Shin HI, Kim JY. Contribution of mesenchymal proliferation in tooth root morphogenesis. J Dent Res. 2014; 93:78–83.13. Bae CH, Kim TH, Chu JY, Cho ES. New population of odontoblasts responsible for tooth root formation. Gene Expr Patterns. 2013; 13:197–202.14. Hardcastle Z, Mo R, Hui CC, Sharpe PT. The Shh signalling pathway in tooth development: defects in Gli2 and Gli3 mutants. Development. 1998; 125:2803–2811.15. Wright T. The molecular control of and clinical variations in root formation. Cells Tissues Organs. 2007; 186:86–93.16. Ten Cate AR. The role of epithelium in the development, structure and function of the tissues of tooth support. Oral Dis. 1996; 2:55–62.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The role of nuclear factor I-C in tooth and bone development
- Changes in the titer of tooth root antibodies accompanying root resorption associated with orthodontic tooth movement
- Pressure Root Resorption of the Second Molar Caused by Third Molar Impaction: A Case Report of Severely Resorbed Root with Vital Pulp
- The relationships between the arrangement of teeth, root resorption, and dental maturity in bovine mandibular incisors
- Attachment and Differentiation of Human Umbilical Cord Stem Cells on to the Tooth Root Surface with and without the Use of Fibroblast Growth Factor-An In Vitro Study