Cancer Res Treat.
2018 Oct;50(4):1433-1443. 10.4143/crt.2017.223.
The NEAT Predictive Model for Survival in Patients with Advanced Cancer
- Affiliations
-
- 1Department of Radiation Oncology, Good Samaritan Hospital Medical Center, West Islip, NY, USA. azucker20@gmail.com
- 2New York Institute of Technology College of Osteopathic Medicine, Old Westbury, NY, USA.
- 3Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
- 4Divisions of Hematology and Medical Oncology, Good Samaritan Hospital Medical Center, West Islip, NY, USA.
- 5Divisions of Supportive and Palliative Care, Good Samaritan Hospital Medical Center, West Islip, NY, USA.
- KMID: 2424813
- DOI: http://doi.org/10.4143/crt.2017.223
Abstract
- PURPOSE
We previously developed a model to more accurately predict life expectancy for stage IV cancer patients referred to radiation oncology. The goals of this study are to validate this model and to compare competing published models.
MATERIALS AND METHODS
From May 2012 to March 2015, 280 consecutive patientswith stage IV cancerwere prospectively evaluated by a single radiation oncologist. Patients were separated into training, validation and combined sets. TheNEAT model evaluated number of active tumors ("N"), Eastern Cooperative Oncology Group performance status ("E"), albumin ("A") and primary tumor site ("T"). The Odette Cancer Center model validated performance status, bone only metastases and primary tumor site. The Harvard TEACHH model investigated primary tumor type, performance status, age, prior chemotherapy courses, liver metastases, and hospitalization within 3 months. Cox multivariable analyses and logisticalregressionwere utilized to compare model performance.
RESULTS
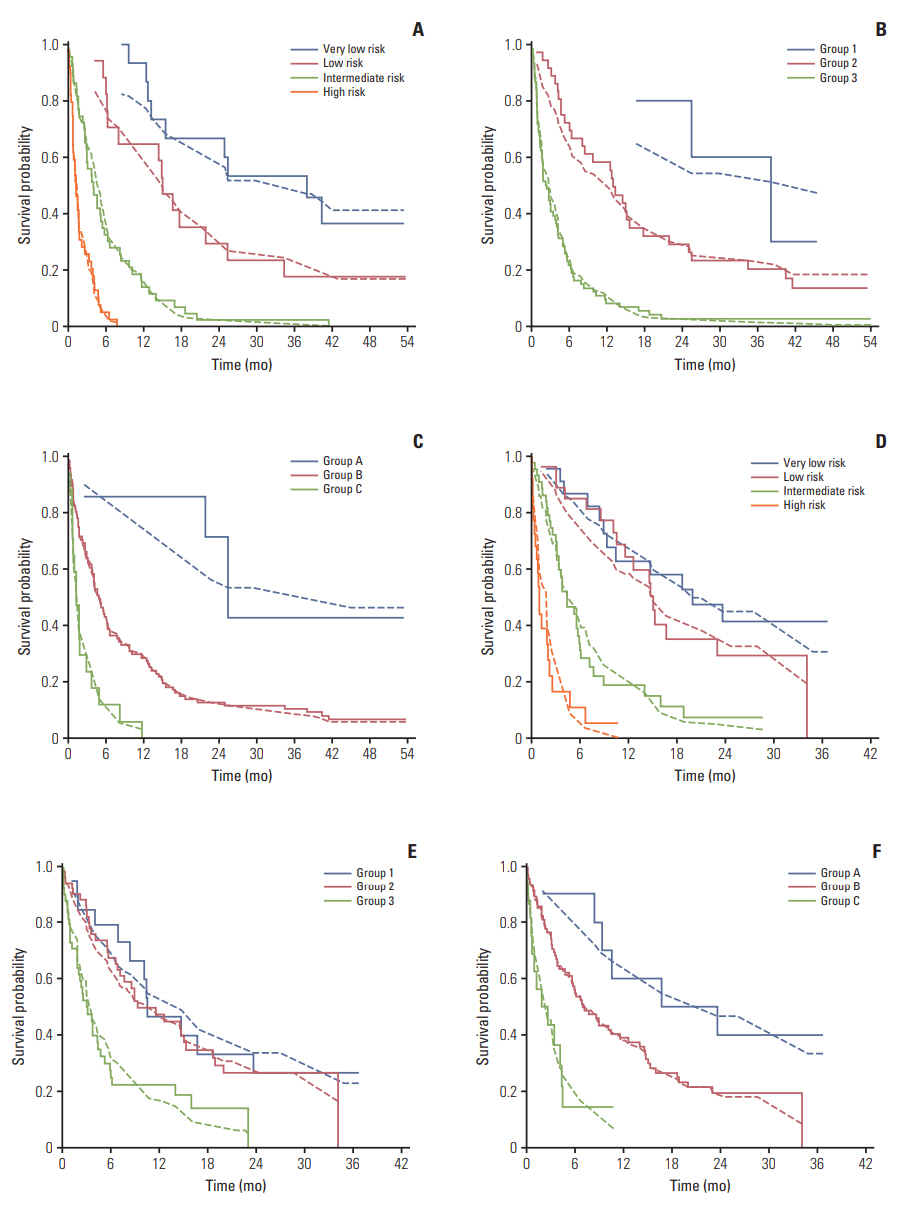
Number of active tumors, performance status, albumin, primary tumor site, prior hospitalizationwithin the last 3 months, and liver metastases predicted overall survival on uinvariate and multivariable analysis (p < 0.05 for all). The NEAT model separated patients into four prognostic groups with median survivals of 24.9, 14.8, 4.0, and 1.2 months, respectively (p < 0.001). The NEAT model had a C-index of 0.76 with a Nagelkerke's R2 of 0.54 suggesting good discrimination, calibration and total performance compared to competing prognostic models.
CONCLUSION
The NEAT model warrants further investigation as a clinically useful approach to predict survival in patients with stage IV cancer.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Jones JA, Lutz ST, Chow E, Johnstone PA. Palliative radiotherapy at the end of life: a critical review. CA Cancer J Clin. 2014; 64:296–310.
Article2. Lamont EB, Christakis NA. Complexities in prognostication in advanced cancer: "to help them live their lives the way they want to". JAMA. 2003; 290:98–104.3. Chow E, Davis L, Panzarella T, Hayter C, Szumacher E, Loblaw A, et al. Accuracy of survival prediction by palliative radiation oncologists. Int J Radiat Oncol Biol Phys. 2005; 61:870–3.
Article4. Hartsell WF, Desilvio M, Bruner DW, Scarantino C, Ivker R, Roach M 3rd, et al. Can physicians accurately predict survival time in patients with metastatic cancer? Analysis of RTOG 97-14. J Palliat Med. 2008; 11:723–8.
Article5. Kao J, Gold KD, Zarrili G, Copel E, Silverman AJ, Ramsaran SS, et al. Clinical predictors of survival for patients with stage IV cancer referred to radiation oncology. PLoS One. 2015; 10:e0124329.6. Chow E, Abdolell M, Panzarella T, Harris K, Bezjak A, Warde P, et al. Predictive model for survival in patients with advanced cancer. J Clin Oncol. 2008; 26:5863–9.
Article7. Krishnan MS, Epstein-Peterson Z, Chen YH, Tseng YD, Wright AA, Temel JS, et al. Predicting life expectancy in patients with metastatic cancer receiving palliative radiotherapy: the TEACHH model. Cancer. 2014; 120:134–41.
Article8. Mack JW, Cronin A, Taback N, Huskamp HA, Keating NL, Malin JL, et al. End-of-life care discussions among patients with advanced cancer: a cohort study. Ann Intern Med. 2012; 156:204–10.9. Morden NE, Chang CH, Jacobson JO, Berke EM, Bynum JP, Murray KM, et al. End-of-life care for medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood). 2012; 31:786–96.
Article10. Baek SK, Chang HJ, Byun JM, Han JJ, Heo DS. The association between end-of-life care and the time interval between provision of a do-not-resuscitate consent and death in cancer patients in Korea. Cancer Res Treat. 2017; 49:502–8.
Article11. Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010; 21:128–38.12. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996; 15:361–87.
Article13. Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012; 307:182–92.14. Newson RB. Comparing the predictive powers of survival models using Harrell's C or Somers' D. Stata J. 2010; 10:339–58.
Article15. Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P, McColl E, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016; 388:2004–14.
Article16. Milano MT, Katz AW, Zhang H, Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term followup of prospective study. Int J Radiat Oncol Biol Phys. 2012; 83:878–86.
Article17. Salama JK, Hasselle MD, Chmura SJ, Malik R, Mehta N, Yenice KM, et al. Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer. 2012; 118:2962–70.18. Gripp S, Mjartan S, Boelke E, Willers R. Palliative radiotherapy tailored to life expectancy in end-stage cancer patients: reality or myth? Cancer. 2010; 116:3251–6.19. Koshy M, Malik R, Mahmood U, Rusthoven CG, Sher DJ. Comparative effectiveness of aggressive thoracic radiation therapy and concurrent chemoradiation therapy in metastatic lung cancer. Pract Radiat Oncol. 2015; 5:374–82.
Article20. Gomez DR, Blumenschein GR Jr, Lee JJ, Hernandez M, Ye R, Camidge DR, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016; 17:1672–82.
Article21. Kao J, Chen CT, Tong CC, Packer SH, Schwartz M, Chen SH, et al. Concurrent sunitinib and stereotactic body radiotherapy for patients with oligometastases: final report of a prospective clinical trial. Target Oncol. 2014; 9:145–53.22. Feliu J, Jimenez-Gordo AM, Madero R, Rodriguez-Aizcorbe JR, Espinosa E, Castro J, et al. Development and validation of a prognostic nomogram for terminally ill cancer patients. J Natl Cancer Inst. 2011; 103:1613–20.
Article23. Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010; 77:655–61.
Article24. Rades D, Fehlauer F, Schulte R, Veninga T, Stalpers LJ, Basic H, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006; 24:3388–93.
Article25. Chow E, James JL, Hartsell W, Scarantino CW, Ivker R, Roach M, et al. Validation of a predictive model for survival in patients with advanced cancer: secondary analysis of RTOG 9714. World J Oncol. 2011; 2:181–90.26. Inui A. Cancer anorexia-cachexia syndrome: current issues in research and management. CA Cancer J Clin. 2002; 52:72–91.
Article27. Kao J, Packer S, Vu HL, Schwartz ME, Sung MW, Stock RG, et al. Phase 1 study of concurrent sunitinib and image-guided radiotherapy followed by maintenance sunitinib for patients with oligometastases: acute toxicity and preliminary response. Cancer. 2009; 115:3571–80.28. Anderson F, Downing GM, Hill J, Casorso L, Lerch N. Palliative performance scale (PPS): a new tool. J Palliat Care. 1996; 12:5–11.
Article29. Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A, et al. Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-mol-GPA). JAMA Oncol. 2017; 3:827–31.30. Chen HM, Ma G, Gildener-Leapman N, Eisenstein S, Coakley BA, Ozao J, et al. Myeloid-derived suppressor cells as an immune parameter in patients with concurrent sunitinib and stereotactic body radiotherapy. Clin Cancer Res. 2015; 21:4073–85.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increaseing Physical Activity in Daily Life
- Does the Retrieval of at Least 15 Lymph Nodes Confer an Improved Survival in Patients with Advanced Gastric Cancer?
- Evaluation and Comparison of Predictive Value of Tumor Regression Grades according to Mandard and Becker in Locally Advanced Gastric Adenocarcinoma
- Potential predictors for chemotherapeutic response and prognosis in epithelial ovarian, fallopian tube and primary peritoneal cancer patients treated with platinum-based chemotherapy
- The Value of the Illness-Death Model for Predicting Outcomes in Patients with Non–Small Cell Lung Cancer