Cancer Res Treat.
2018 Oct;50(4):1362-1377. 10.4143/crt.2017.353.
Preclinical Study of Novel Curcumin Analogue SSC-5 Using Orthotopic Tumor Xenograft Model for Esophageal Squamous Cell Carcinoma
- Affiliations
-
- 1Department of Surgery, The University of Hong Kong, Hong Kong. nikkilee@hku.hk yjhxhc@mail.sysu.edu.cn
- 2Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Zhejiang University, Hangzhou, China.
- 3School of Chemical Engineering and Technology, Sun Yat-Sen University, Guangzhou, China. nikkilee@hku.hk yjhxhc@mail.sysu.edu.cn
- 4Guangdong Petrochemical Research Institute, Guangzhou, China.
- 5Guangxi Teachers Education University, College of Chemistry and Materials Science, Nanning, China.
- KMID: 2424808
- DOI: http://doi.org/10.4143/crt.2017.353
Abstract
- PURPOSE
Tumor xenograft model is an indispensable animal cancer model. In esophageal squamous cell carcinoma (ESCC) research, orthotopic tumor xenograft model establishes tumor xenograft in the animal esophagus, which allows the study of tumorigenesis in its native microenvironment.
MATERIALS AND METHODS
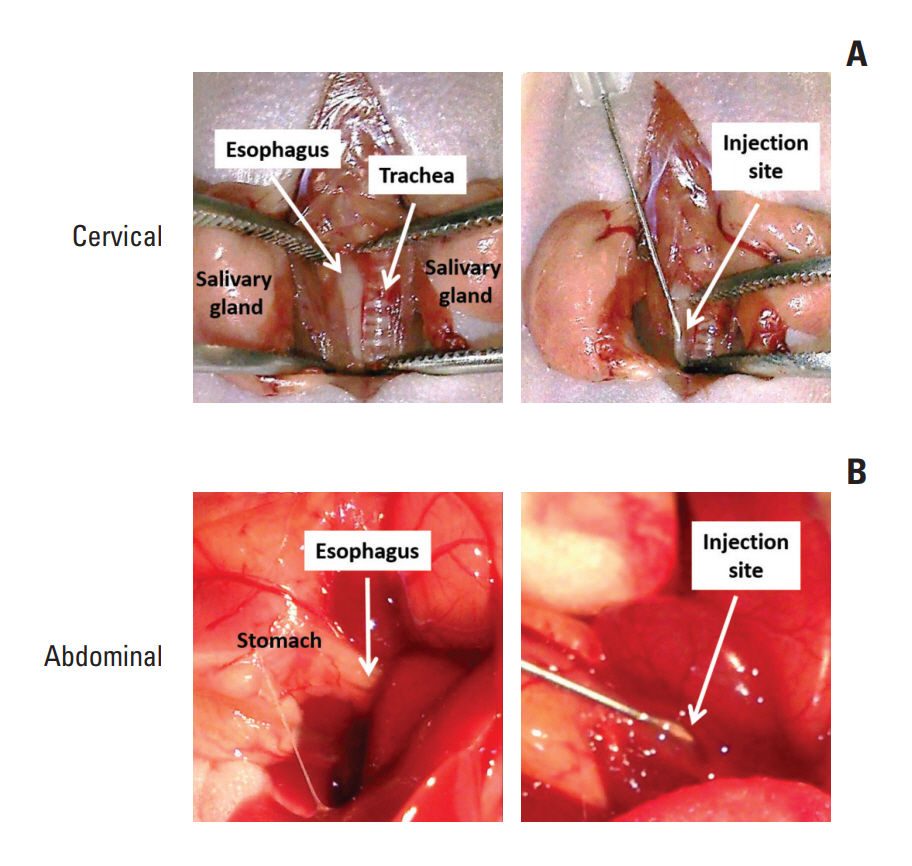
In this study,we described two simple and reproducible methods to develop tumor xenograft at the cervical or the abdominal esophagus in nude mice by direct injection of ESCC cells in the esophageal wall.
RESULTS
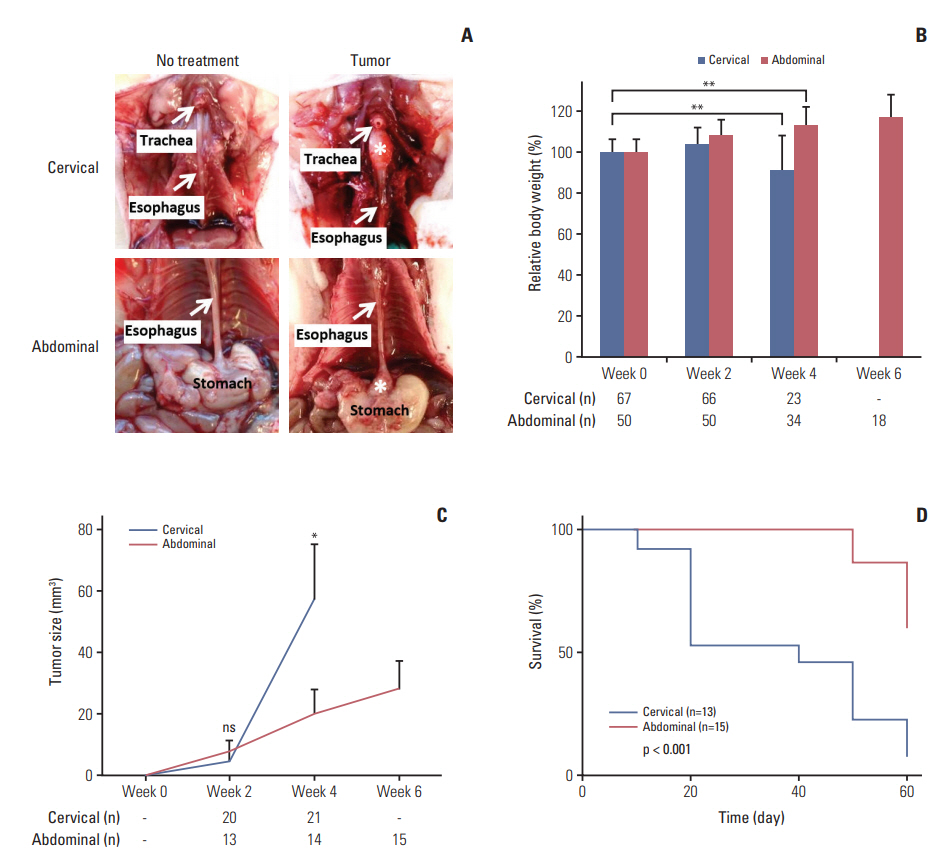
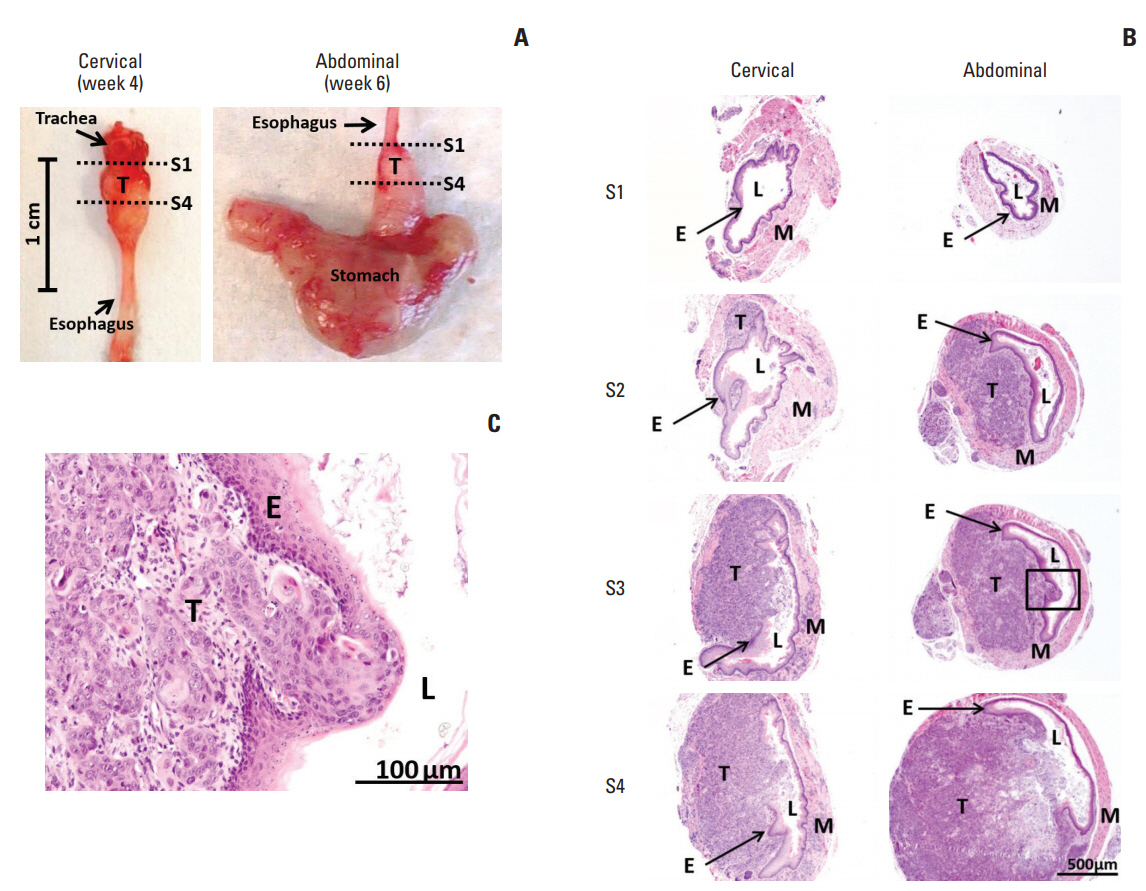
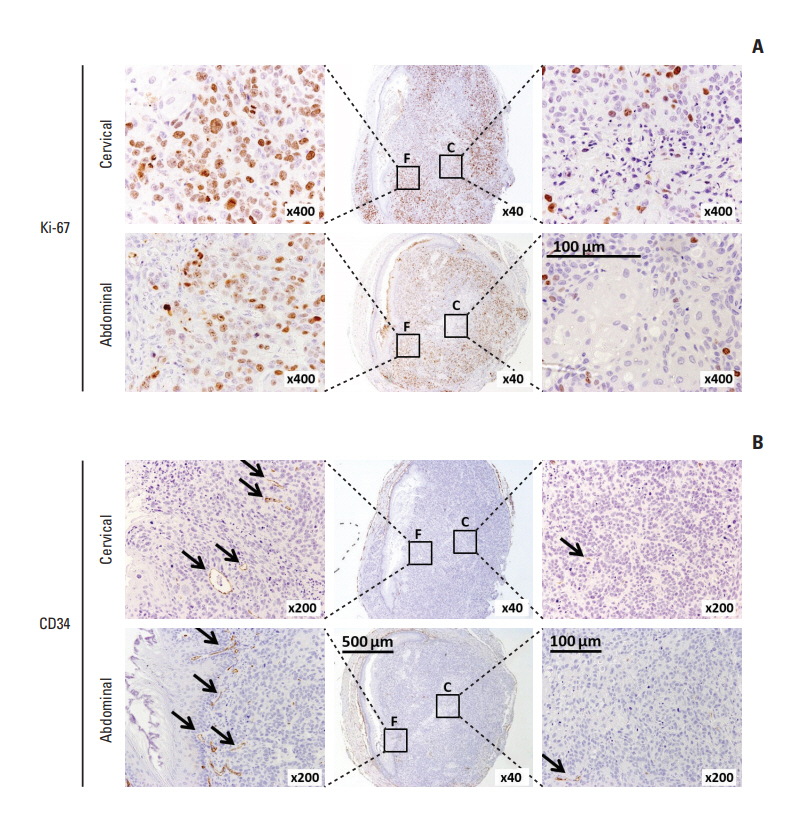
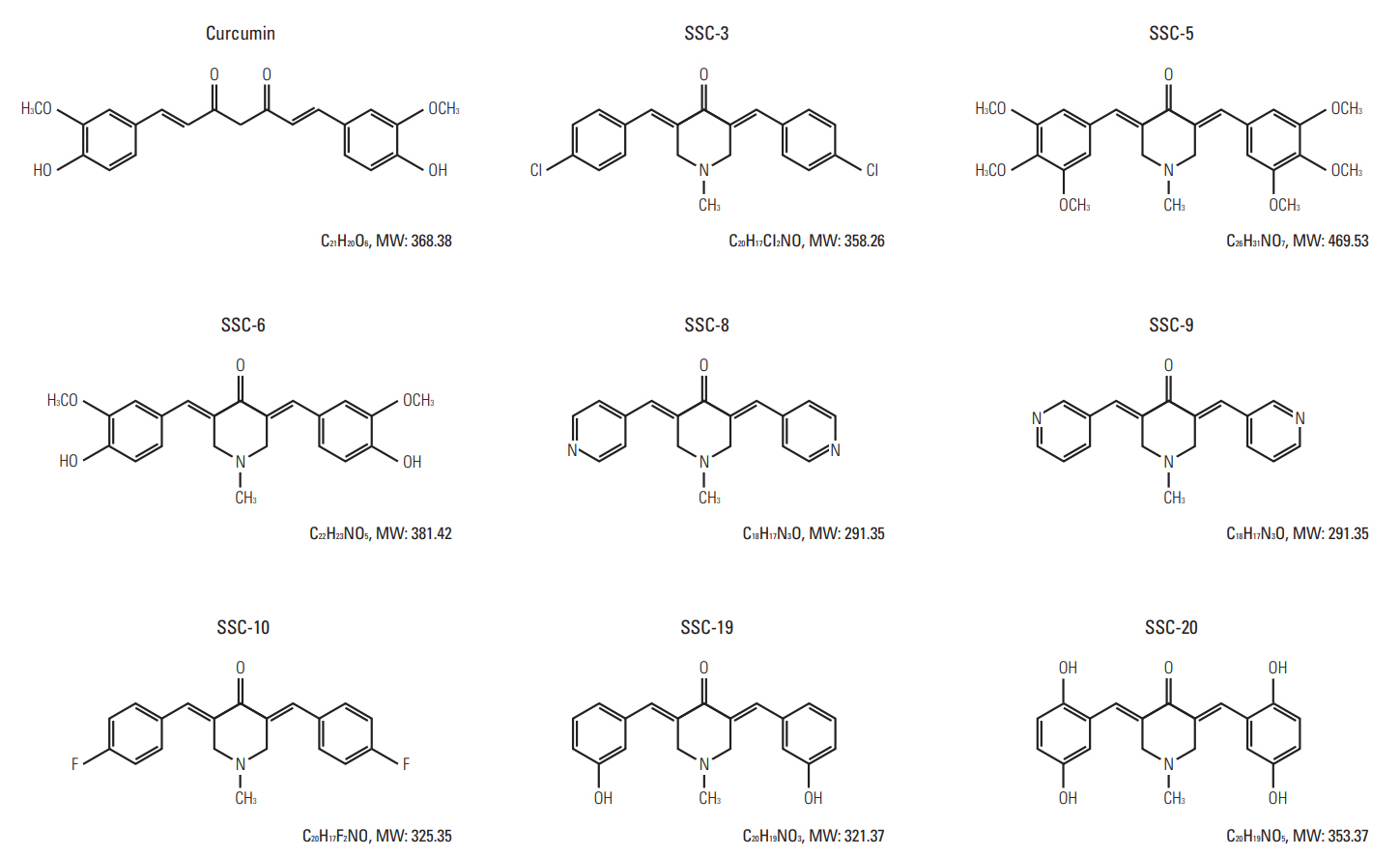
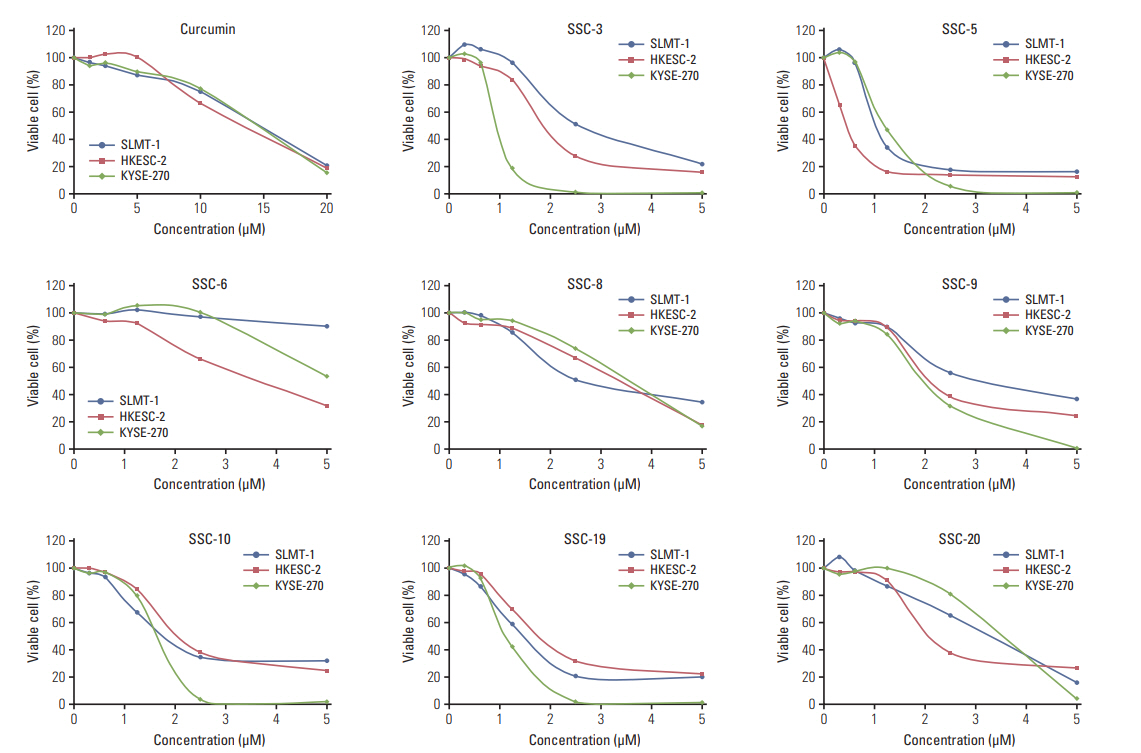
In comparing these two methods, the cervical one presented with more clinically relevant features, i.e., esophageal stricture, body weight loss and poor survival. In addition, the derived tumor xenografts accompanied a rapid growth rate and a high tendency to invade into the surrounding structures. This model was subsequently used to study the anti-tumor effect of curcumin, which is known for its potential therapeutic effects in various diseases including cancers, and its analogue SSC-5. SSC-5 was selected among the eight newly synthesized curcumin analogues based on its superior anti-tumor effect demonstrated in an MTT cell proliferation assay and its effects on apoptosis induction and cell cycle arrest in cultured ESCC cells. Treatment of orthotopic tumor-bearing mice with SSC-5 resulted in an inhibition in tumor growth and invasion.
CONCLUSION
Taken together, we have established a clinically relevant orthotopic tumor xenograft model that can serve as a preclinical tool for screening new anti-tumor compounds, e.g., SSC-5, in ESCC.
MeSH Terms
Figure
Reference
-
References
1. Killion JJ, Radinsky R, Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev. 1998; 17:279–84.2. Fidler IJ, Wilmanns C, Staroselsky A, Radinsky R, Dong Z, Fan D. Modulation of tumor cell response to chemotherapy by the organ environment. Cancer Metastasis Rev. 1994; 13:209–22.
Article3. Dong Z, Radinsky R, Fan D, Tsan R, Bucana CD, Wilmanns C, et al. Organ-specific modulation of steady-state mdr gene expression and drug resistance in murine colon cancer cells. J Natl Cancer Inst. 1994; 86:913–20.
Article4. Hori T, Yamashita Y, Ohira M, Matsumura Y, Muguruma K, Hirakawa K. A novel orthotopic implantation model of human esophageal carcinoma in nude rats: CD44H mediates cancer cell invasion in vitro and in vivo. Int J Cancer. 2001; 92:489–96.5. Ohara T, Takaoka M, Sakurama K, Nagaishi K, Takeda H, Shirakawa Y, et al. The establishment of a new mouse model with orthotopic esophageal cancer showing the esophageal stricture. Cancer Lett. 2010; 293:207–12.
Article6. Kuroda S, Kubota T, Aoyama K, Kikuchi S, Tazawa H, Nishizaki M, et al. Establishment of a non-invasive semi-quantitative bioluminescent imaging method for monitoring of an orthotopic esophageal cancer mouse model. PLoS One. 2014; 9:e114562.
Article7. Song S, Chang D, Cui Y, Hu J, Gong M, Ma K, et al. New orthotopic implantation model of human esophageal squamous cell carcinoma in athymic nude mice. Thorac Cancer. 2014; 5:417–24.
Article8. Hu T, Qi H, Li P, Zhao G, Ma Y, Hao Q, et al. Comparison of GFP-expressing imageable mouse models of human esophageal squamous cell carcinoma established in various anatomical sites. Anticancer Res. 2015; 35:4655–63.9. Ip JC, Ko JM, Yu VZ, Chan KW, Lam AK, Law S, et al. A versatile orthotopic nude mouse model for study of esophageal squamous cell carcinoma. Biomed Res Int. 2015; 2015:910715.
Article10. Padhye S, Chavan D, Pandey S, Deshpande J, Swamy KV, Sarkar FH. Perspectives on chemopreventive and therapeutic potential of curcumin analogs in medicinal chemistry. Mini Rev Med Chem. 2010; 10:372–87.
Article11. Bandyopadhyay D. Farmer to pharmacist: curcumin as an anti-invasive and antimetastatic agent for the treatment of cancer. Front Chem. 2014; 2:113.
Article12. Wei X, Du ZY, Cui XX, Verano M, Mo RQ, Tang ZK, et al. Effects of cyclohexanone analogues of curcumin on growth, apoptosis and NF-kappaB activity in PC-3 human prostate cancer cells. Oncol Lett. 2012; 4:279–84.13. Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007; 67:3853–61.14. Seo JA, Kim B, Dhanasekaran DN, Tsang BK, Song YS. Curcumin induces apoptosis by inhibiting sarco/endoplasmic reticulum Ca2+ ATPase activity in ovarian cancer cells. Cancer Lett. 2016; 371:30–7.
Article15. Liu Z, Xie Z, Jones W, Pavlovicz RE, Liu S, Yu J, et al. Curcumin is a potent DNA hypomethylation agent. Bioorg Med Chem Lett. 2009; 19:706–9.
Article16. Nagaraju GP, Zhu S, Wen J, Farris AB, Adsay VN, Diaz R, et al. Novel synthetic curcumin analogues EF31 and UBS109 are potent DNA hypomethylating agents in pancreatic cancer. Cancer Lett. 2013; 341:195–203.
Article17. Meiyanto E, Putri DD, Susidarti RA, Murwanti R, Sardjiman , Fitriasari A, et al. Curcumin and its analogues (PGV-0 and PGV-1) enhance sensitivity of resistant MCF-7 cells to doxorubicin through inhibition of HER2 and NF-kB activation. Asian Pac J Cancer Prev. 2014; 15:179–84.
Article18. Lai KK, Chan KT, Choi MY, Wang HK, Fung EY, Lam HY, et al. 14-3-3sigma confers cisplatin resistance in esophageal squamous cell carcinoma cells via regulating DNA repair molecules. Tumour Biol. 2016; 37:2127–36.19. Lee NP, Chan KT, Choi MY, Lam HY, Tung LN, Tzang FC, et al. Oxygen carrier YQ23 can enhance the chemotherapeutic drug responses of chemoresistant esophageal tumor xenografts. Cancer Chemother Pharmacol. 2015; 76:1199–207.
Article20. Costi R, Di Santo R, Artico M, Miele G, Valentini P, Novellino E, et al. Cinnamoyl compounds as simple molecules that inhibit p300 histone acetyltransferase. J Med Chem. 2007; 50:1973–7.
Article21. Wei X, Du ZY, Zheng X, Cui XX, Conney AH, Zhang K. Synthesis and evaluation of curcumin-related compounds for anticancer activity. Eur J Med Chem. 2012; 53:235–45.
Article22. Yadav B, Taurin S, Rosengren RJ, Schumacher M, Diederich M, Somers-Edgar TJ, et al. Synthesis and cytotoxic potential of heterocyclic cyclohexanone analogues of curcumin. Bioorg Med Chem. 2010; 18:6701–7.
Article23. Bazzaro M, Anchoori RK, Mudiam MK, Issaenko O, Kumar S, Karanam B, et al. α,β-Unsaturated carbonyl system of chalcone-based derivatives is responsible for broad inhibition of proteasomal activity and preferential killing of human papilloma virus (HPV) positive cervical cancer cells. J Med Chem. 2011; 54:449–56.
Article24. Qiu X, Liu Z, Shao WY, Liu X, Jing DP, Yu YJ, et al. Synthesis and evaluation of curcumin analogues as potential thioredoxin reductase inhibitors. Bioorg Med Chem. 2008; 16:8035–41.
Article25. Chan KT, Choi MY, Lai KK, Tan W, Tung LN, Lam HY, et al. Overexpression of transferrin receptor CD71 and its tumorigenic properties in esophageal squamous cell carcinoma. Oncol Rep. 2014; 31:1296–304.
Article26. Zhang L, Zhang X, Barrisford GW, Olumi AF. Lexatumumab (TRAIL-receptor 2 mAb) induces expression of DR5 and promotes apoptosis in primary and metastatic renal cell carcinoma in a mouse orthotopic model. Cancer Lett. 2007; 251:146–57.
Article27. Abou-Elkacem L, Arns S, Brix G, Gremse F, Zopf D, Kiessling F, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther. 2013; 12:1322–31.
Article28. Wilmanns C, Fan D, Obrian C, Radinsky R, Bucana C, Tsan R, et al. Modulation of doxorubicin sensitivity and level of p-glycoprotein expression in human colon-carcinoma cells by ectopic and orthotopic environments in nude-mice. Int J Oncol. 1993; 3:413–22.
Article29. Kuo TH, Kubota T, Watanabe M, Furukawa T, Kase S, Tanino H, et al. Site-specific chemosensitivity of human small-cell lung carcinoma growing orthotopically compared to subcutaneously in SCID mice: the importance of orthotopic models to obtain relevant drug evaluation data. Anticancer Res. 1993; 13:627–30.30. Jiang YJ, Lee CL, Wang Q, Zhou ZW, Yang F, Jin C, et al. Establishment of an orthotopic pancreatic cancer mouse model: cells suspended and injected in Matrigel. World J Gastroenterol. 2014; 20:9476–85.
Article31. Quatromoni JG, Predina JD, Bhojnagarwala P, Judy RP, Jiang J, De Jesus EM, et al. Adenoviral-based immunotherapy provides local disease control in an orthotopic murine model of esophageal cancer. J Immunother. 2014; 37:283–92.
Article32. Tian F, Zhang C, Tian W, Jiang Y, Zhang X. Comparison of the effect of p65 siRNA and curcumin in promoting apoptosis in esophageal squamous cell carcinoma cells and in nude mice. Oncol Rep. 2012; 28:232–40.
Article33. Qiu X, Du Y, Lou B, Zuo Y, Shao W, Huo Y, et al. Synthesis and identification of new 4-arylidene curcumin analogues as potential anticancer agents targeting nuclear factor-kappaB signaling pathway. J Med Chem. 2010; 53:8260–73.34. Zhou J, Geng G, Shi Q, Sauriol F, Wu JH. Design and synthesis of androgen receptor antagonists with bulky side chains for overcoming antiandrogen resistance. J Med Chem. 2009; 52:5546–50.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Animal Models of Cancer in the Head and Neck Region
- Cutaneous Metastasis of Esophageal Squamous Cell Carcinoma Mimicking Benign Soft Tissue Tumor
- The Generation and Application of Patient-Derived Xenograft Model for Cancer Research
- Basaloid-Squamous Carcinoma of the Esophagus: A case report
- A Case of Squamous Cell Carcinoma Metastasized from Esophageal Carcinoma