Cancer Res Treat.
2018 Oct;50(4):1331-1342. 10.4143/crt.2017.466.
High-Dose Metformin Plus Temozolomide Shows Increased Anti-tumor Effects in Glioblastoma In Vitro and In vivo Compared with Monotherapy
- Affiliations
-
- 1Department of Neurosurgery, St. Vincent's Hospital, Cell Death Disease Research Center, College of Medicine, The Catholic University of Korea, Suwon, Korea. 72ysh@catholic.ac.kr
- 2Division of Nephrology, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Neurosurgery, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2424805
- DOI: http://doi.org/10.4143/crt.2017.466
Abstract
- PURPOSE
The purpose of the study is to investigate the efficacy of combined treatment with temozolomide (TMZ) and metformin for glioblastoma (GBM) in Vitro and in vivo.
MATERIALS AND METHODS
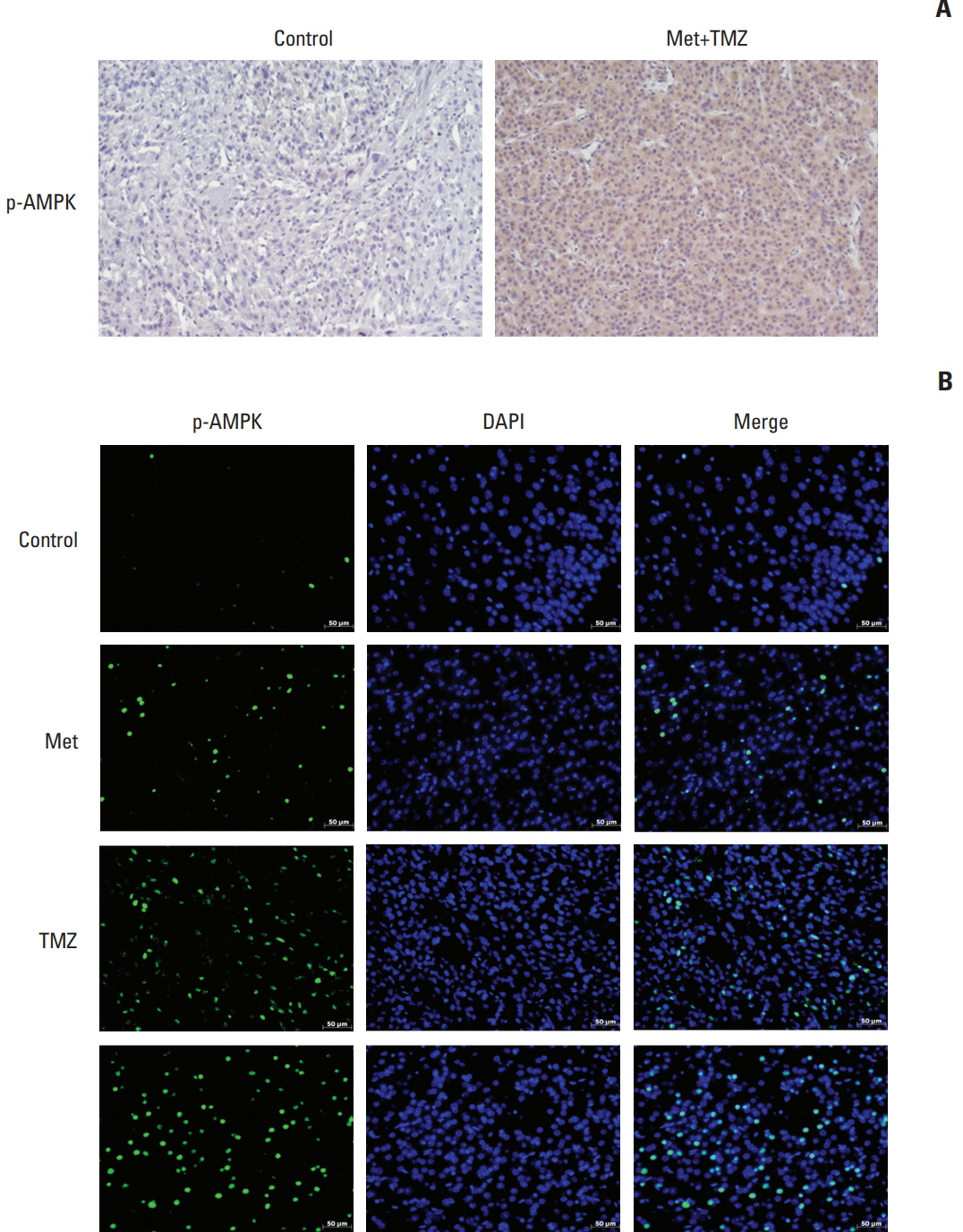
We investigated the efficacy of combined treatment with TMZ and metformin using cell viability and apoptosis assays. A GBM orthotopic mice model was established by inoculation of 5×105 U87 cells and treatedwith metformin, TMZ, and the combination for 4weeks. Western blotting and immunofluorescence of tumor specimens were analyzed to investigate AMP-activated protein kinase (AMPK) and AKT pathway.
RESULTS
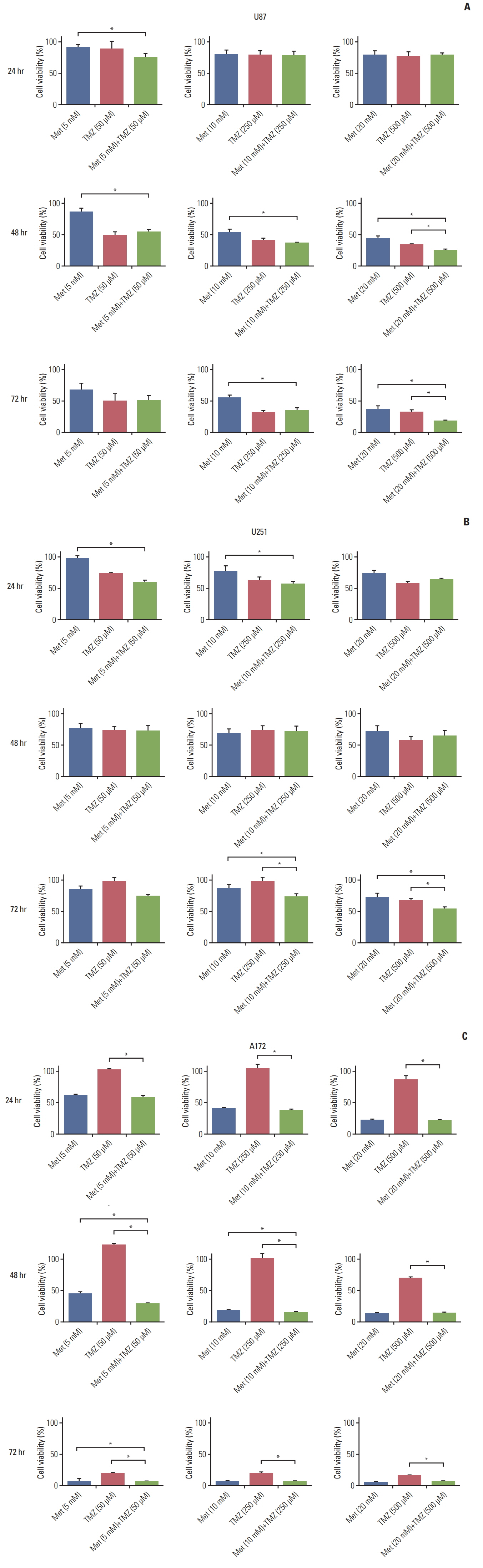
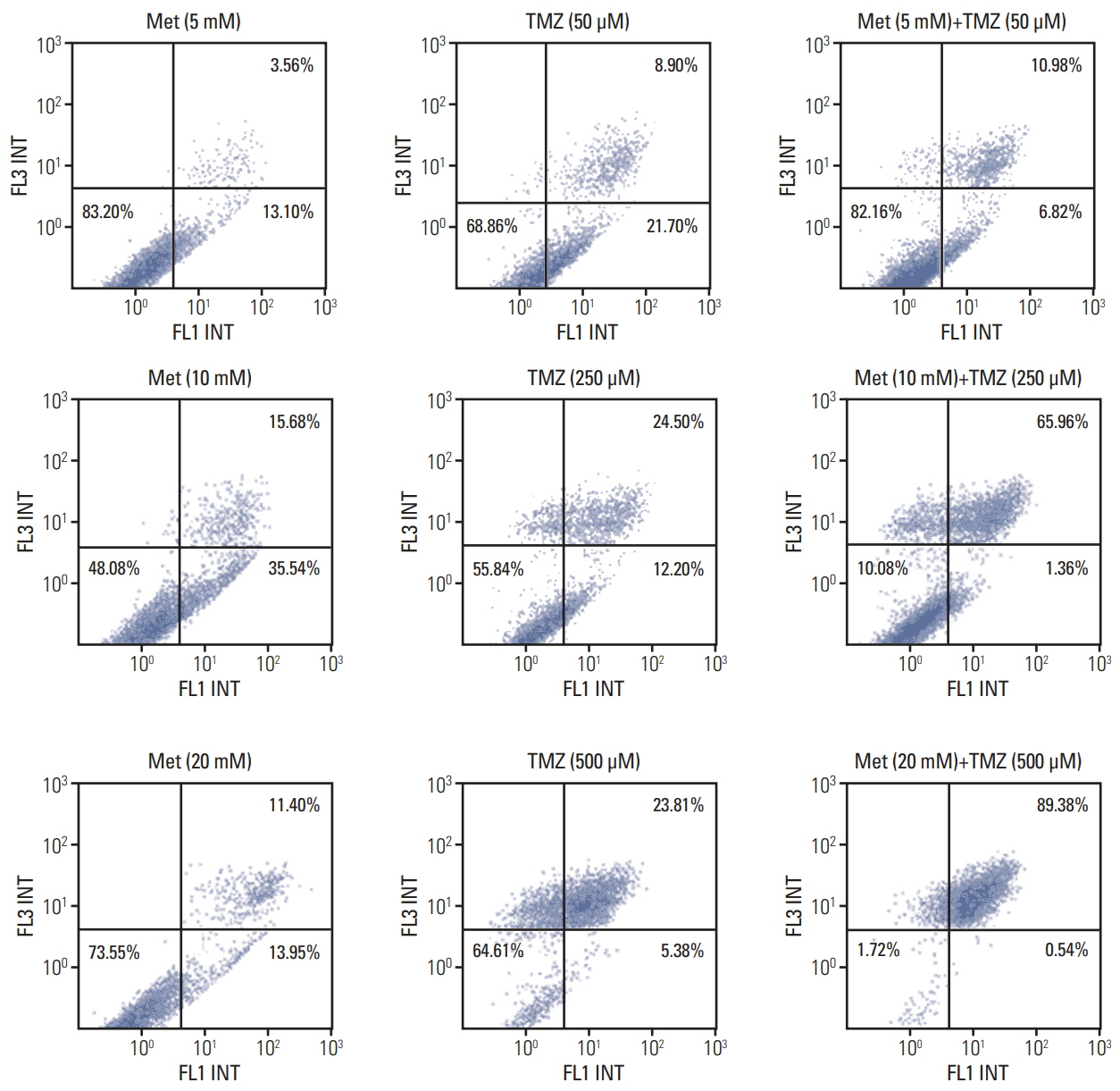
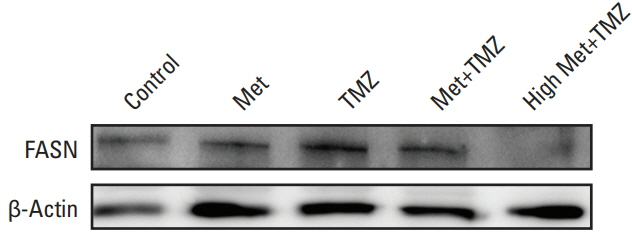
The combination of TMZ and metformin showed higher cytotoxicity than single agents in U87, U251, and A172 cell lines. A combination of high-dose metformin and TMZ showed the highest apoptotic activity. The combination of TMZ and metformin enhanced AMPK phosphorylation and inhibited mammalian target of rapamycin phosphorylation, AKT phosphorylation, and p53 expression. The median survival of each group was 43.6, 55.2, 53.2, 65.2, and 71.3 days for control, metformin treatment (2 mg/25 g/day or 10 mg/25 g/day), TMZ treatment (15 mg/kg/day), combination treatment with low-dose metformin and TMZ, and combination treatment with high-dose metformin and TMZ, respectively. Expression of fatty acid synthase (FASN) was significantly decreased in tumor specimens treated with metformin and TMZ.
CONCLUSION
The combination of metformin and TMZ was superior to monotherapy using metformin or TMZ in terms of cell viability in Vitro and survival in vivo. The combination of high-dose metformin and TMZ inhibited FASN expression in an orthotopic model. Inhibition of FASN might be a potential therapeutic target of GBM.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Kyritsis AP, Levin VA. An algorithm for chemotherapy treatment of recurrent glioma patients after temozolomide failure in the general oncology setting. Cancer Chemother Pharmacol. 2011; 67:971–83.
Article2. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–96.
Article3. Di Cristofori A, Carrabba G, Lanfranchi G, Menghetti C, Rampini P, Caroli M. Continuous tamoxifen and dose-dense temozolomide in recurrent glioblastoma. Anticancer Res. 2013; 33:3383–9.4. Zustovich F, Landi L, Lombardi G, Porta C, Galli L, Fontana A, et al. Sorafenib plus daily low-dose temozolomide for relapsed glioblastoma: a phase II study. Anticancer Res. 2013; 33:3487–94.5. Saran F, Chinot OL, Henriksson R, Mason W, Wick W, Cloughesy T, et al. Bevacizumab, temozolomide, and radiotherapy for newly diagnosed glioblastoma: comprehensive safety results during and after first-line therapy. Neuro Oncol. 2016; 18:991–1001.
Article6. Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009; 69:7507–11.
Article7. Gou S, Cui P, Li X, Shi P, Liu T, Wang C. Low concentrations of metformin selectively inhibit CD133(+) cell proliferation in pancreatic cancer and have anticancer action. PLoS One. 2013; 8:e63969.
Article8. Nangia-Makker P, Yu Y, Vasudevan A, Farhana L, Rajendra SG, Levi E, et al. Metformin: a potential therapeutic agent for recurrent colon cancer. PLoS One. 2014; 9:e84369.
Article9. Shank JJ, Yang K, Ghannam J, Cabrera L, Johnston CJ, Reynolds RK, et al. Metformin targets ovarian cancer stem cells in Vitro and in vivo. Gynecol Oncol. 2012; 127:390–7.10. Yu Z, Zhao G, Li P, Li Y, Zhou G, Chen Y, et al. Temozolomide in combination with metformin act synergistically to inhibit proliferation and expansion of glioma stem-like cells. Oncol Lett. 2016; 11:2792–800.
Article11. Yu Z, Zhao G, Xie G, Zhao L, Chen Y, Yu H, et al. Metformin and temozolomide act synergistically to inhibit growth of glioma cells and glioma stem cells in Vitro and in vivo. Oncotarget. 2015; 6:32930–43.12. Sesen J, Dahan P, Scotland SJ, Saland E, Dang VT, Lemarie A, et al. Metformin inhibits growth of human glioblastoma cells and enhances therapeutic response. PLoS One. 2015; 10:e0123721.
Article13. Bosi E. Metformin: the gold standard in type 2 diabetes: what does the evidence tell us? Diabetes Obes Metab. 2009; 11 Suppl 2:3–8.14. Cerezo M, Tichet M, Abbe P, Ohanna M, Lehraiki A, Rouaud F, et al. Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner. Mol Cancer Ther. 2013; 12:1605–15.
Article15. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016; 7:27–31.
Article16. Kordes S, Pollak MN, Zwinderman AH, Mathot RA, Weterman MJ, Beeker A, et al. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015; 16:839–47.
Article17. Wakil SJ. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry. 1989; 28:4523–30.
Article18. Seguin F, Carvalho MA, Bastos DC, Agostini M, Zecchin KG, Alvarez-Flores MP, et al. The fatty acid synthase inhibitor orlistat reduces experimental metastases and angiogenesis in B16-F10 melanomas. Br J Cancer. 2012; 107:977–87.
Article19. Zaytseva YY, Rychahou PG, Gulhati P, Elliott VA, Mustain WC, O'Connor K, et al. Inhibition of fatty acid synthase attenuates CD44-associated signaling and reduces metastasis in colorectal cancer. Cancer Res. 2012; 72:1504–17.
Article20. Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007; 7:763–77.
Article21. Grube S, Dunisch P, Freitag D, Klausnitzer M, Sakr Y, Walter J, et al. Overexpression of fatty acid synthase in human gliomas correlates with the WHO tumor grade and inhibition with Orlistat reduces cell viability and triggers apoptosis. J Neurooncol. 2014; 118:277–87.
Article22. Zhou Y, Jin G, Mi R, Zhang J, Zhang J, Xu H, et al. Inhibition of fatty acid synthase suppresses neovascularization via regulating the expression of VEGF-A in glioma. J Cancer Res Clin Oncol. 2016; 142:2447–59.
Article23. Yasumoto Y, Miyazaki H, Vaidyan LK, Kagawa Y, Ebrahimi M, Yamamoto Y, et al. Inhibition of fatty acid synthase decreases expression of stemness markers in glioma stem cells. PLoS One. 2016; 11:e0147717.
Article24. Yang SH, Li S, Lu G, Xue H, Kim DH, Zhu JJ, et al. Metformin treatment reduces temozolomide resistance of glioblastoma cells. Oncotarget. 2016; 7:78787–803.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glioblastoma in a Patient with Neurofibromatosis Type 1: A Case Report and Review of the Literature
- Temozolomide Drives Ferroptosis via a DMT1-Dependent Pathway in Glioblastoma Cells
- Temozolomide-Associated Bronchiolitis Obliterans Organizing Pneumonia Successfully Treated with High-Dose Corticosteroid
- The Effect of Metformin in Obese Pediatric Patients with Type 2 Diabetes
- Effects of Metformin on Hepatic Steatosis in Adults with Nonalcoholic Fatty Liver Disease and Diabetes: Insights from the Cellular to Patient Levels