Cancer Res Treat.
2018 Oct;50(4):1324-1330. 10.4143/crt.2017.526.
Multicenter Phase II Study of Oxaliplatin, Irinotecan, and S-1 as First-line Treatment for Patients with Recurrent or Metastatic Biliary Tract Cancer
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. ryooby@amc.seoul.kr
- 2Department of Internal Medicine, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea. fhdzang@hallym.ac.kr
- 3Asan Institute for Life Science, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 5Department of Radiology, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea.
- 6Department of Pathology, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea.
- KMID: 2424804
- DOI: http://doi.org/10.4143/crt.2017.526
Abstract
- PURPOSE
Although gemcitabine plus cisplatin has been established as the standard first-line chemotherapy for patients with advanced biliary tract cancer (BTC), overall prognosis remains poor. We investigated the efficacy of a novel triplet combination of oxaliplatin, irinotecan, and S-1 (OIS) for advanced BTC.
MATERIALS AND METHODS
Chemotherapy-naive patientswith histologically documented unresectable or metastatic BTC were eligible for this multicenter, single-arm phase II study. Patients received 65 mg/m2 oxaliplatin (day 1), 135 mg/m2 irinotecan (day 1), and 40 mg/m2 S-1 (twice a day, days 1-7) every 2 weeks. Primary endpoint was objective response rate. Targeted exome sequencing for biomarker analysis was performed using archival tissue.
RESULTS
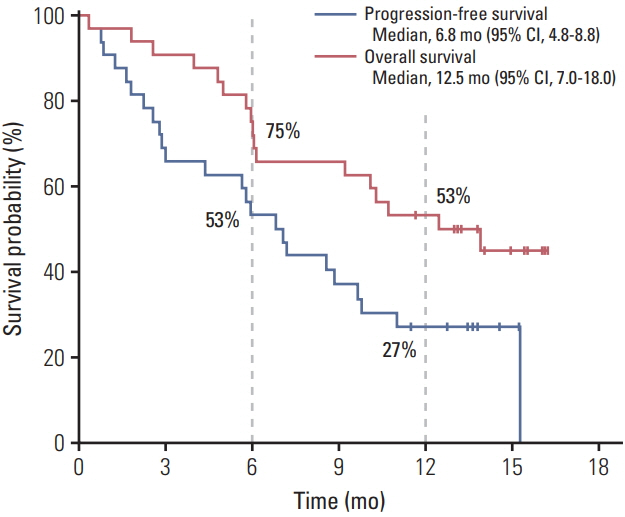
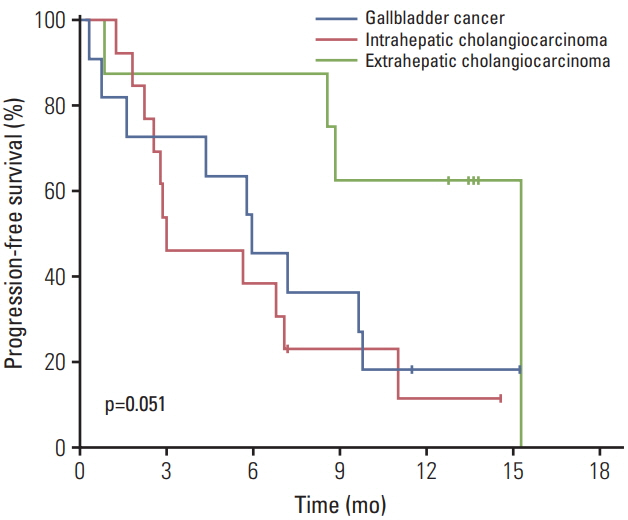
In total, 32 patients were enrolled between October 2015 and June 2016. Median age was 64 years (range, 40 to 76 years), with 24 (75%) male patients; 97% patients had metastatic or recurrent disease. Response rate was 50%, and median progression-free survival and overall survival (OS) were 6.8 months (95% confidence interval [CI], 4.8 to 8.8) and 12.5 months (95% CI, 7.0 to 18.0), respectively. The most common grade 3-4 adverse events were neutropenia (32%), diarrhea (6%), and peripheral neuropathy (6%). TP53 and KRAS mutations were the most frequent genomic alterations (42% and 32%, respectively), and KRAS mutations showed a marginal relationship with worse OS (p=0.07).
CONCLUSION
OIS combination chemotherapy was feasible and associated with favorable efficacy outcomes as a first-line treatment in patients with advanced BTC. Randomized studies are needed to compare OIS with gemcitabine plus cisplatin.
MeSH Terms
Figure
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30.
Article2. Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH. Prediction of cancer incidence and mortality in Korea, 2017. Cancer Res Treat. 2017; 49:306–12.
Article3. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–4.
Article4. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010; 362:1273–81.
Article5. Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010; 103:469–74.
Article6. Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006; 24:4991–7.
Article7. Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011; 364:1817–25.
Article8. Han B, Jung JY, Kim HS, Cho JW, Kim KC, Lim H, et al. A dose-finding study for oxaliplatin, irinotecan, and S-1 (OIS) in patients with metastatic or recurrent gastrointestinal cancer. Cancer Chemother Pharmacol. 2016; 78:949–58.
Article9. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article10. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989; 10:1–10.
Article11. Kim BJ, Hyung J, Yoo C, Kim KP, Park SJ, Lee SS, et al. Prognostic factors in patients with advanced biliary tract cancer treated with first-line gemcitabine plus cisplatin: retrospective analysis of 740 patients. Cancer Chemother Pharmacol. 2017; 80:209–15.
Article12. Robertson S, Hyder O, Dodson R, Nayar SK, Poling J, Beierl K, et al. The frequency of KRAS and BRAF mutations in intrahepatic cholangiocarcinomas and their correlation with clinical outcome. Hum Pathol. 2013; 44:2768–73.13. Churi CR, Shroff R, Wang Y, Rashid A, Kang HC, Weatherly J, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One. 2014; 9:e115383.
Article14. Kim BJ, Yoo C, Kim KP, Hyung J, Park SJ, Ryoo BY, et al. Efficacy of fluoropyrimidine-based chemotherapy in patients with advanced biliary tract cancer after failure of gemcitabine plus cisplatin: retrospective analysis of 321 patients. Br J Cancer. 2017; 116:561–7.
Article15. Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015; 47:1003–10.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current status of chemotherapy for the treatment of advanced biliary tract cancer
- Chemotherapy for Colorecal Cancer
- Drug-induced immune-mediated thrombocytopenia due to bevacizumab-FOLFOX therapy: a case report
- Oxaliplatin and Leucovorin Plus Fluorouracil Versus Irinotecan and Leucovorin Plus Fluorouracil Combination Chemotherapy as a First-line Treatment in Patients with Metastatic or Recurred Gastric Adenocarcinoma
- Novel Palliative Chemotherapy for Cholangiocarcinoma