Cancer Res Treat.
2018 Oct;50(4):1304-1315. 10.4143/crt.2017.463.
Induction Chemotherapy Plus Concurrent Chemoradiotherapy Versus Concurrent Chemoradiotherapy Alone in Locoregionally Advanced Nasopharyngeal Carcinoma in Children and Adolescents: A Matched Cohort Analysis
- Affiliations
-
- 1Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China. maihq@sysucc.org.cn gzzhaochong@hotmail.com chenqy@sysucc.org.cn
- 2Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, China.
- 3Department of Radiation Oncology, Sun Yat-Sen University Cancer Center, Guangzhou, China.
- KMID: 2424802
- DOI: http://doi.org/10.4143/crt.2017.463
Abstract
- PURPOSE
The purpose of this study was to evaluate the long-term clinical outcome and toxicity of induction chemotherapy (IC) followed by concomitant chemoradiotherapy (CCRT) compared with CCRT alone for the treatment of children and adolescent locoregionally advanced nasopharyngeal carcinoma (LACANPC).
MATERIALS AND METHODS
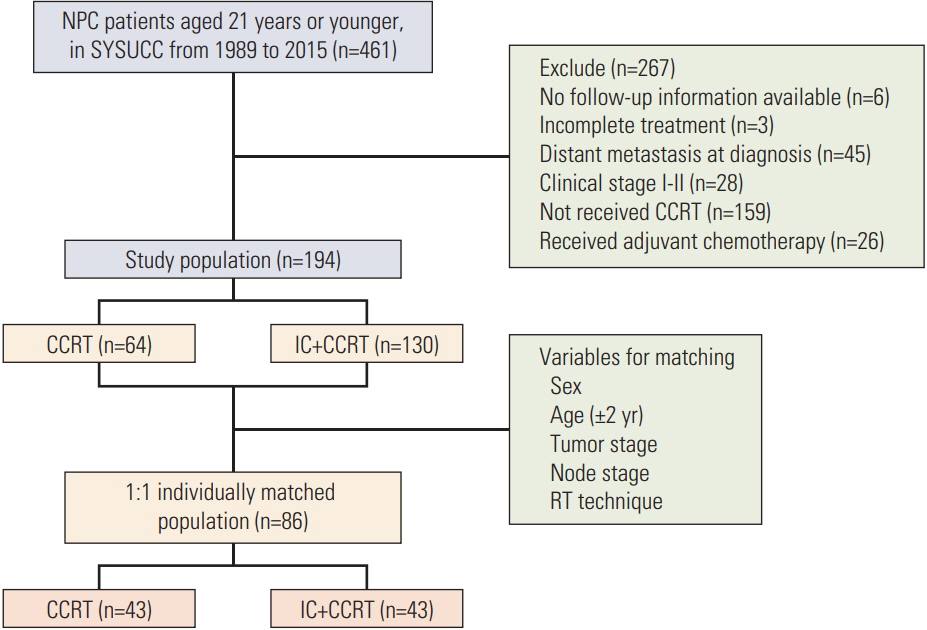
A total of 194 locoregionally advanced nasopharyngeal carcinoma patients youngerthan 21 years who received CCRT with or without IC before were included in the study population. Overall survival (OS) rate, progression-free survival (PFS) rate, locoregional recurrence-free survival (LRFS) rate, and distant metastasis-free survival (DMFS) rate were assessed by the Kaplan-Meier method and a log-rank test. Treatment toxicities were clarified and compared between two groups.
RESULTS
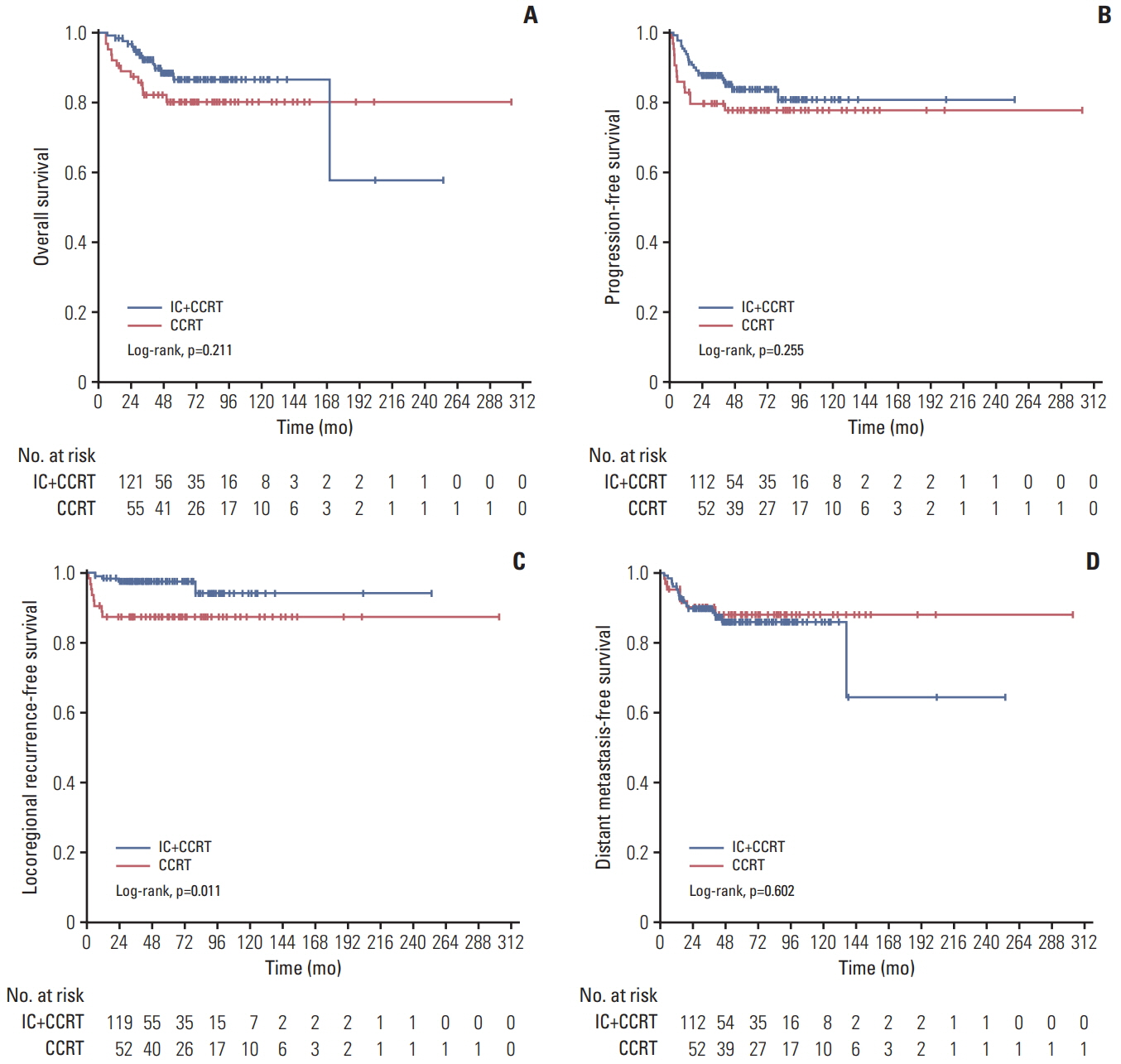
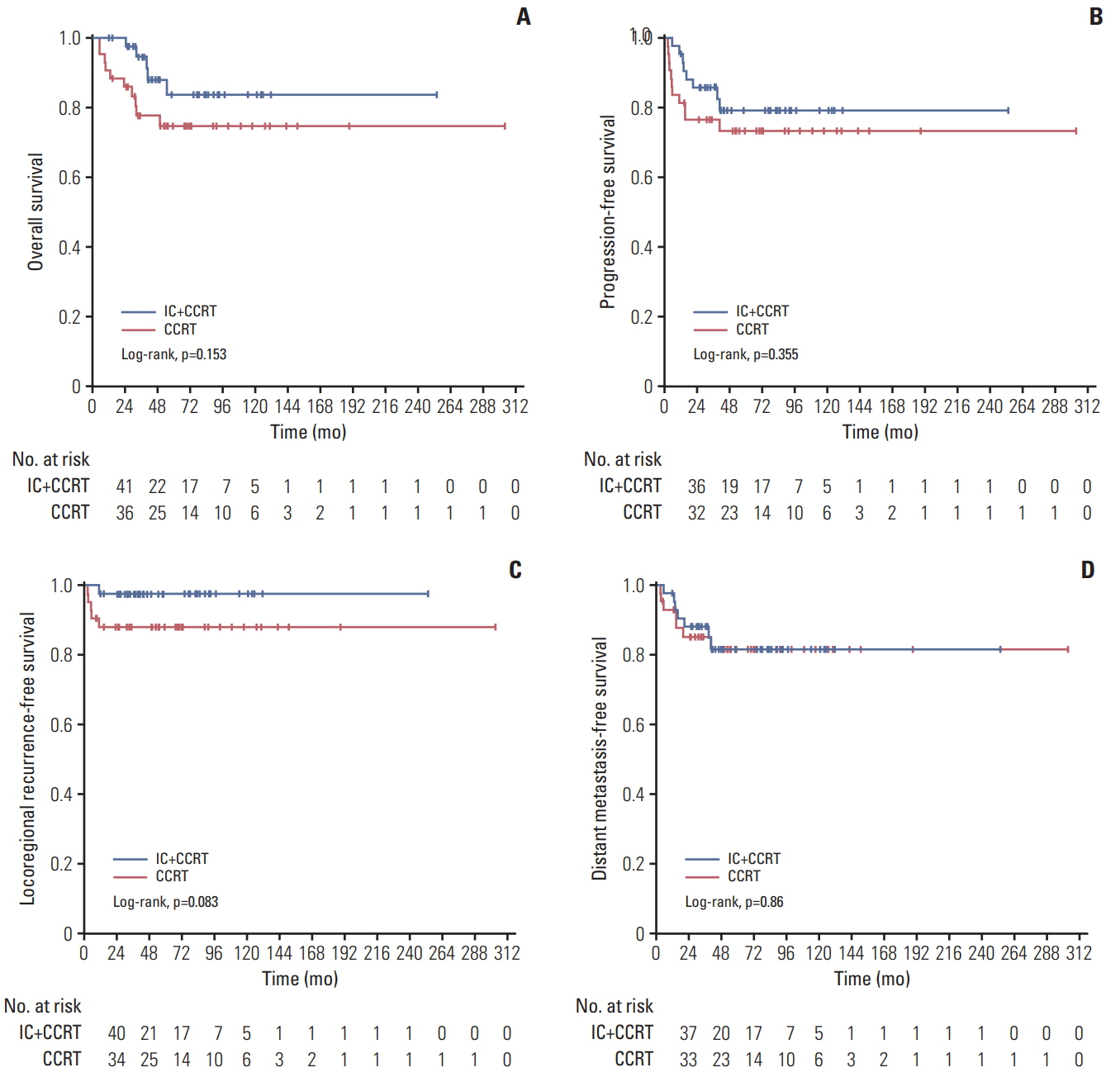
One hundred and thiry of 194 patients received IC+CCRT. Patients who were younger and with more advanced TNM stage were more likely to receive IC+CCRT and intensive modulated radiotherapy. The addition of IC before CCRT failed to improve survival significantly. The matched analysis identified 43 well-balanced patients in both two groups. With a median follow-up of 51.5 months, no differences were found between the IC+CCRT group and the CCRT group in 5-year OS (83.7% vs. 74.6%, p=0.153), PFS (79.2% vs. 73.4%, p=0.355), LRFS (97.7% vs. 88.2%, p=0.083), and DMFS (81.6% vs. 81.6%, p=0.860). N3 was an independent prognostic factor predicting poorer OS, PFS, and DMFS. The addition of IC was associated with increased rates of grade 3 to 4 neutropenia.
CONCLUSION
This study failed to demonstrate that adding IC before CCRT could provide a significant additional survival benefit for LACANPC patients. Further investigations are warranted.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005; 365:2041–54.
Article2. Levine PH, Connelly RR, Easton JM. Demographic patterns for nasopharyngeal carcinoma in the United States. Int J Cancer. 1980; 26:741–8.
Article3. Ingersoll L, Woo SY, Donaldson S, Giesler J, Maor MH, Goffinet D, et al. Nasopharyngeal carcinoma in the young: a combined M.D. Anderson and Stanford experience. Int J Radiat Oncol Biol Phys. 1990; 19:881–7.
Article4. Downing NL, Wolden S, Wong P, Petrik DW, Hara W, Le QT. Comparison of treatment results between adult and juvenile nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2009; 75:1064–70.
Article5. Yan Z, Xia L, Huang Y, Chen P, Jiang L, Zhang B. Nasopharyngeal carcinoma in children and adolescents in an endemic area: a report of 185 cases. Int J Pediatr Otorhinolaryngol. 2013; 77:1454–60.
Article6. Guo Q, Cui X, Lin S, Lin J, Pan J. Locoregionally advanced nasopharyngeal carcinoma in childhood and adolescence: Analysis of 95 patients treated with combined chemotherapy and intensity-modulated radiotherapy. Head Neck. 2016; 38 Suppl 1:E665–72.
Article7. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016; 17:1509–20.8. Ribassin-Majed L, Marguet S, Lee AW, Ng WT, Ma J, Chan AT, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network meta-analysis. J Clin Oncol. 2017; 35:498–505.
Article9. Casanova M, Ozyar E, Patte C, Orbach D, Ferrari A, Veyrat-Follet C, et al. International randomized phase 2 study on the addition of docetaxel to the combination of cisplatin and 5-fluorouracil in the induction treatment for nasopharyngeal carcinoma in children and adolescents. Cancer Chemother Pharmacol. 2016; 77:289–98.
Article10. Rodriguez-Galindo C, Krailo MD, Krasin MJ, McCarville MB, Hicks J, Pashankar F, et al. Treatment of childhood nasopharyngeal carcinoma (cNPC) with neoadjuvant chemotherapy (NAC) and concomitant chemoradiotherapy (CCRT): results of the Children’s Oncology Group ARAR0331 study. J Clin Oncol. 2016; 34(15 Suppl):Abstr.
Article11. Zaghloul MS, Eldebawy E, Ahmed S, Ammar H, Khalil E, Abdelrahman H, et al. Does primary tumor volume predict the outcome of pediatric nasopharyngeal carcinoma?: A prospective single-arm study using neoadjuvant chemotherapy and concomitant chemotherapy with intensity modulated radiotherapy. Asia Pac J Clin Oncol. 2016; 12:143–50.
Article12. Varan A, Ozyar E, Corapcioglu F, Koksal Y, Aydin B, Yazici N, et al. Pediatric and young adult nasopharyngeal carcinoma patients treated with preradiation cisplatin and docetaxel chemotherapy. Int J Radiat Oncol Biol Phys. 2009; 73:1116–20.
Article13. Xiao WW, Huang SM, Han F, Wu SX, Lu LX, Lin CG, et al. Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer. 2011; 117:1874–83.14. Ma J, Mai HQ, Hong MH, Cui NJ, Lu TX, Lu LX, et al. Is the 1997 AJCC staging system for nasopharyngeal carcinoma prognostically useful for Chinese patient populations? Int J Radiat Oncol Biol Phys. 2001; 50:1181–9.
Article15. Zhao C, Han F, Lu LX, Huang SM, Lin CG, Deng XW, et al. Intensity modulated radiotherapy for local-regional advanced nasopharyngeal carcinoma. Ai Zheng. 2004; 23(11 Suppl):1532–7.16. Ma J, Liu L, Tang L, Zong J, Lin A, Lu T, et al. Retropharyngeal lymph node metastasis in nasopharyngeal carcinoma: prognostic value and staging categories. Clin Cancer Res. 2007; 13:1445–52.
Article17. Ozyar E, Selek U, Laskar S, Uzel O, Anacak Y, Ben-Arush M, et al. Treatment results of 165 pediatric patients with nonmetastatic nasopharyngeal carcinoma: a Rare Cancer Network study. Radiother Oncol. 2006; 81:39–46.
Article18. Bakkal BH, Kaya B, Berberoglu S, Aksu G, Sayin MY, Altundag MB, et al. The efficiency of different chemoradiotherapy regimens in patients with paediatric nasopharynx cancer: review of 46 cases. Int J Clin Pract. 2007; 61:52–61.
Article19. Lu S, Chang H, Sun X, Zhen Z, Sun F, Zhu J, et al. Long-term outcomes of nasopharyngeal carcinoma in 148 children and adolescents. Medicine (Baltimore). 2016; 95:e3445.
Article20. Orbach D, Brisse H, Helfre S, Klijanienko J, Bours D, Mosseri V, et al. Radiation and chemotherapy combination for nasopharyngeal carcinoma in children: Radiotherapy dose adaptation after chemotherapy response to minimize late effects. Pediatr Blood Cancer. 2008; 50:849–53.
Article21. Tao CJ, Liu X, Tang LL, Mao YP, Chen L, Li WF, et al. Longterm outcome and late toxicities of simultaneous integrated boost-intensity modulated radiotherapy in pediatric and adolescent nasopharyngeal carcinoma. Chin J Cancer. 2013; 32:525–32.
Article22. Zubizarreta PA, D'Antonio G, Raslawski E, Gallo G, Preciado MV, Casak SJ, et al. Nasopharyngeal carcinoma in childhood and adolescence: a single-institution experience with combined therapy. Cancer. 2000; 89:690–5.23. Cheuk DK, Billups CA, Martin MG, Roland CR, Ribeiro RC, Krasin MJ, et al. Prognostic factors and long-term outcomes of childhood nasopharyngeal carcinoma. Cancer. 2011; 117:197–206.
Article24. Rodriguez-Galindo C, Wofford M, Castleberry RP, Swanson GP, London WB, Fontanesi J, et al. Preradiation chemotherapy with methotrexate, cisplatin, 5-fluorouracil, and leucovorin for pediatric nasopharyngeal carcinoma. Cancer. 2005; 103:850–7.
Article25. Cannon T, Zanation AM, Lai V, Weissler MC. Nasopharyngeal carcinoma in young patients: a systematic review of racial demographics. Laryngoscope. 2006; 116:1021–6.
Article26. Casanova M, Bisogno G, Gandola L, Cecchetto G, Di Cataldo A, Basso E, et al. A prospective protocol for nasopharyngeal carcinoma in children and adolescents: the Italian Rare Tumors in Pediatric Age (TREP) project. Cancer. 2012; 118:2718–25.27. Tang LQ, Chen QY, Fan W, Liu H, Zhang L, Guo L, et al. Prospective study of tailoring whole-body dual-modality [18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol. 2013; 31:2861–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Less is more: role of additional chemotherapy to concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal cancer management
- Organ Preservation for the Management of Locally Advanced Head and Neck Cancer
- Does the Addition of Adjuvant Chemotherapy to Concurrent Chemoradiotherapy Improve the Survival of Patients with Locally Advanced Nasopharyngeal Cancer?
- Locoregionally advanced nasopharyngeal carcinoma treated with intensity-modulated radiotherapy plus concurrent weekly cisplatin with or without neoadjuvant chemotherapy
- The Addition of Induction Chemotherapy Failed to Improve Therapeutic Outcome of Concurrent Chemoradiotherapy in Patients with Locally Advanced Non-small Cell Lung Cancer