Cancer Res Treat.
2018 Oct;50(4):1294-1303. 10.4143/crt.2017.512.
The Association of Acquired T790M Mutation with Clinical Characteristics after Resistance to First-Line Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor in Lung Adenocarcinoma
- Affiliations
-
- 1Division of Chest Medicine, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan. august@vghtc.gov.tw, jonyin@gmail.com
- 2Division of Critical Care and Respiratory Therapy, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan.
- 3Institute of Biomedical Sciences, National Chung Hsing University, Taichung, Taiwan.
- 4Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei, Taiwan.
- 5Department of Clinical Laboratory Sciences and Medical Biotechnology, College of Medicine, National Taiwan University, Taipei, Taiwan.
- 6Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan.
- 7Graduate Institute of Toxicology, National Taiwan University, Taipei, Taiwan.
- 8Center of Genomic Medicine, National Taiwan University College of Medicine, Taipei, Taiwan.
- 9Department of Pathology and Graduate Institute of Pathology, College of Medicine, National Taiwan University, Taipei, Taiwan.
- 10Center for Optoelectronic Biomedicine, College of Medicine, National Taiwan University, Taipei, Taiwan.
- 11Comprehensive Cancer Center, Taichung Veterans General Hospital, Taichung, Taiwan.
- KMID: 2424801
- DOI: http://doi.org/10.4143/crt.2017.512
Abstract
- PURPOSE
The main objective of this study was to investigate the relationship among the clinical characteristics and the frequency of T790M mutation in advanced epidermal growth factor receptor (EGFR)-mutant lung adenocarcinoma patients with acquired resistance after firstline EGFR-tyrosine kinase inhibitor (TKI) treatment.
MATERIALS AND METHODS
We enrolled EGFR-mutant stage IIIB-IV lung adenocarcinoma patients, who had progressed to prior EGFR-TKI therapy, and evaluated their rebiopsy EGFR mutation status.
RESULTS
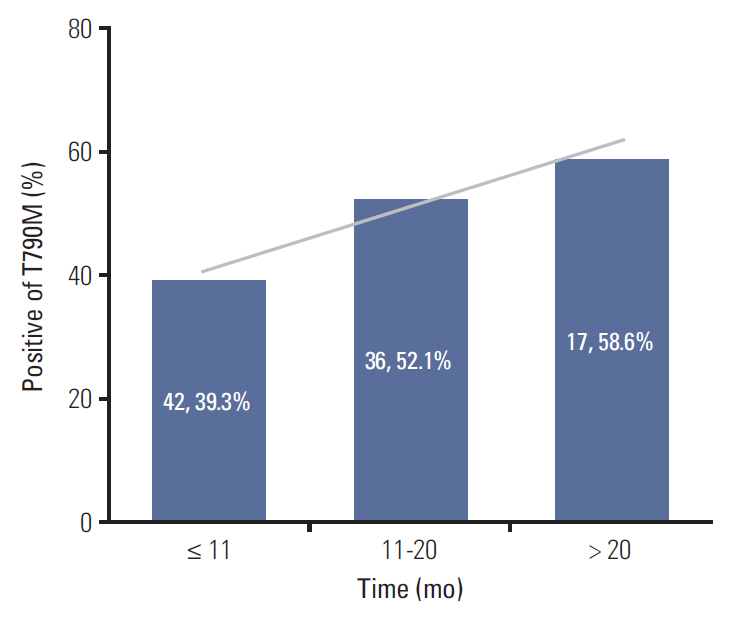
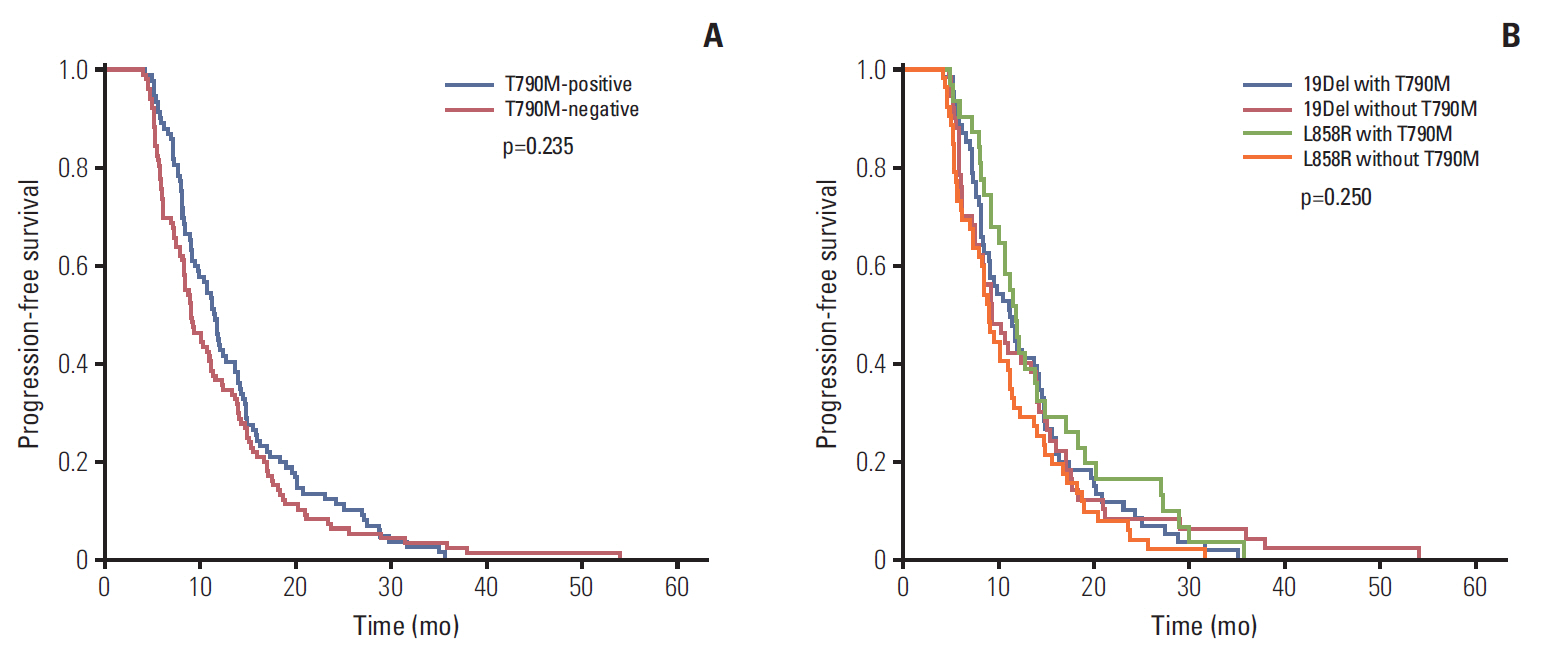
A total of 205 patients were enrolled for analysis. The overall T790M mutation rate of rebiopsy was 46.3%. The T790M mutation rates among patients with exon 19 deletion mutation, exon 21 L858R point mutation, and other mutations were 55.0%, 37.3%, and 27.3%, respectively. Baseline exon 19 deletion was associated with a significantly higher frequency of T790M mutation (adjusted odds ratio, 2.14; 95% confidence interval [CI], 1.20 to 3.83; p=0.010). In the exon 19 deletion subgroup, there was a greater prevalence of T790M mutation than other exon 19 deletion subtypes in patients with the Del E746-A750 mutation (61.6% vs. 40.6%; odds ratio, 2.35; 95% CI, 1.01 to 5.49; p=0.049). The progression-free survival (PFS) of first-line TKI treatment > 11 months was also associated with a higher T790M mutation rate (54.1% vs. 39.3%; adjusted odds ratio, 1.82; 95% CI, 1.02 to 3.25; p=0.044). Patients who underwent rebiopsy at metastatic sites had more chance to harbor T790M mutation (52.6% vs. 33.8%; adjusted odds ratio, 1.97; 95% CI, 1.06 to 3.67; p=0.032).
CONCLUSION
PFS of first-line EGFR-TKI, rebiopsy site, EGFR exon 19 deletion and its subtype Del E746-A750 mutation are associated with the frequency of T790M mutation.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Rare Mechanism of Acquired Resistance to Osimertinib in Korean Patients with
EGFR -mutated Non-small Cell Lung Cancer
Jiyun Lee, Joon Ho Shim, Woong-Yang Park, Hee Kyung Kim, Jong-Mu Sun, Se-Hoon Lee, Jin Seok Ahn, Keunchil Park, Myung-Ju Ahn
Cancer Res Treat. 2019;51(1):408-412. doi: 10.4143/crt.2018.138.The Clinical Outcomes of Different First-Line EGFR
- TKIs Plus Bevacizumab in AdvancedEGFR -Mutant Lung Adenocarcinoma
Yen-Hsiang Huang, Kuo-Hsuan Hsu, Chun-Shih Chin, Jeng-Sen Tseng, Tsung-Ying Yang, Kun-Chieh Chen, Kang-Yi Su, Sung-Liang Yu, Jeremy J.W. Chen, Gee-Chen Chang
Cancer Res Treat. 2022;54(2):434-444. doi: 10.4143/crt.2021.671.
Reference
-
References
1. Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014; 9:154–62.
Article2. Hsu KH, Ho CC, Hsia TC, Tseng JS, Su KY, Wu MF, et al. Identification of five driver gene mutations in patients with treatment-naive lung adenocarcinoma in Taiwan. PLoS One. 2015; 10:e0120852.3. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011; 12:735–42.
Article4. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012; 13:239–46.5. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010; 362:2380–8.
Article6. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010; 11:121–8.
Article7. Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013; 31:3327–34.
Article8. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–39.
Article9. Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004; 304:1497–500.10. Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013; 19:2240–7.
Article11. Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005; 352:786–92.12. Ohashi K, Maruvka YE, Michor F, Pao W. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol. 2013; 31:1070–80.
Article13. Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011; 3:75ra26.
Article14. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017; 376:629–40.
Article15. Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015; 372:1689–99.
Article16. Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014; 4:1046–61.
Article17. Nosaki K, Satouchi M, Kurata T, Yoshida T, Okamoto I, Katakami N, et al. Re-biopsy status among non-small cell lung cancer patients in Japan: a retrospective study. Lung Cancer. 2016; 101:1–8.
Article18. Matsuo N, Azuma K, Sakai K, Hattori S, Kawahara A, Ishii H, et al. Association of EGFR exon 19 deletion and EGFR-TKI treatment duration with frequency of T790M mutation in EGFR-mutant lung cancer patients. Sci Rep. 2016; 6:36458.
Article19. Oya Y, Yoshida T, Kuroda H, Shimizu J, Horio Y, Sakao Y, et al. Association between EGFR T790M status and progression patterns during initial EGFR-TKI treatment in patients harboring EGFR mutation. Clin Lung Cancer. 2017; 18:698–705.
Article20. Tseng JS, Su KY, Yang TY, Chen KC, Hsu KH, Chen HY, et al. The emergence of T790M mutation in EGFR-mutant lung adenocarcinoma patients having a history of acquired resistance to EGFR-TKI: focus on rebiopsy timing and long-term existence of T790M. Oncotarget. 2016; 7:48059–69.
Article21. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–4.
Article22. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article23. Lee VH, Tin VP, Choy TS, Lam KO, Choi CW, Chung LP, et al. Association of exon 19 and 21 EGFR mutation patterns with treatment outcome after first-line tyrosine kinase inhibitor in metastatic non-small-cell lung cancer. J Thorac Oncol. 2013; 8:1148–55.
Article24. Chung KP, Wu SG, Wu JY, Yang JC, Yu CJ, Wei PF, et al. Clinical outcomes in non-small cell lung cancers harboring different exon 19 deletions in EGFR. Clin Cancer Res. 2012; 18:3470–7.
Article25. Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006; 125:1137–49.
Article26. Bose R, Zhang X. The ErbB kinase domain: structural perspectives into kinase activation and inhibition. Exp Cell Res. 2009; 315:649–58.
Article27. Cadranel J, Zalcman G, Sequist L. Genetic profiling and epidermal growth factor receptor-directed therapy in non-small cell lung cancer. Eur Respir J. 2011; 37:183–93.
Article28. Jekunen AP. Role of rebiopsy in relapsed non-small cell lung cancer for directing oncology treatments. J Oncol. 2015; 2015:809835.
Article29. Kim E, Feldman R, Wistuba II. Update on EGFR mutational testing and the potential of noninvasive liquid biopsy in non-small-cell lung cancer. Clin Lung Cancer. 2018; 19:105–14.
Article30. Wu SG, Liu YN, Tsai MF, Chang YL, Yu CJ, Yang PC, et al. The mechanism of acquired resistance to irreversible EGFR tyrosine kinase inhibitor-afatinib in lung adenocarcinoma patients. Oncotarget. 2016; 7:12404–13.
Article31. Tanaka K, Nosaki K, Otsubo K, Azuma K, Sakata S, Ouchi H, et al. Acquisition of the T790M resistance mutation during afatinib treatment in EGFR tyrosine kinase inhibitor-naive patients with non-small cell lung cancer harboring EGFR mutations. Oncotarget. 2017; 8:68123–30.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Basis of Drug Resistance: Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors and Anaplastic Lymphoma Kinase Inhibitors
- Mechanisms of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Resistance and Strategies to Overcome Resistance in Lung Adenocarcinoma
- Mechanisms of Acquired Resistance to Epidermal Growth Factor Receptor Inhibitors and Overcoming Strategies in Lung Cancer
- Treatment of Non-small Cell Lung Carcinoma after Failure of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor
- Repeated Favorable Responses to Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors in a Case of Advanced Lung Adenocarcinoma