Cancer Res Treat.
2018 Oct;50(4):1140-1148. 10.4143/crt.2017.508.
A Novel Prognostic Nomogram for Predicting Risks of Distant Failure in Patients with Invasive Breast Cancer Following Postoperative Adjuvant Radiotherapy
- Affiliations
-
- 1Department of Radiation Oncology, Seoul National University Bundang Hospital, Seongnam, Korea. inah228@snu.ac.kr
- 2Breast Care Center, Seoul National University Bundang Hospital, Seoul National College of Medicine, Seongnam, Korea.
- KMID: 2424787
- DOI: http://doi.org/10.4143/crt.2017.508
Abstract
- PURPOSE
This study aimed to identify predictors for distant metastatic behavior and build a related prognostic nomogram in breast cancer.
MATERIALS AND METHODS
A total of 1,181 patients with non-metastatic breast cancer between 2003 and 2011 were analyzed. To predict the probability of distant metastasis, a nomogram was constructed based on prognostic factors identified using a Cox proportional hazards model.
RESULTS
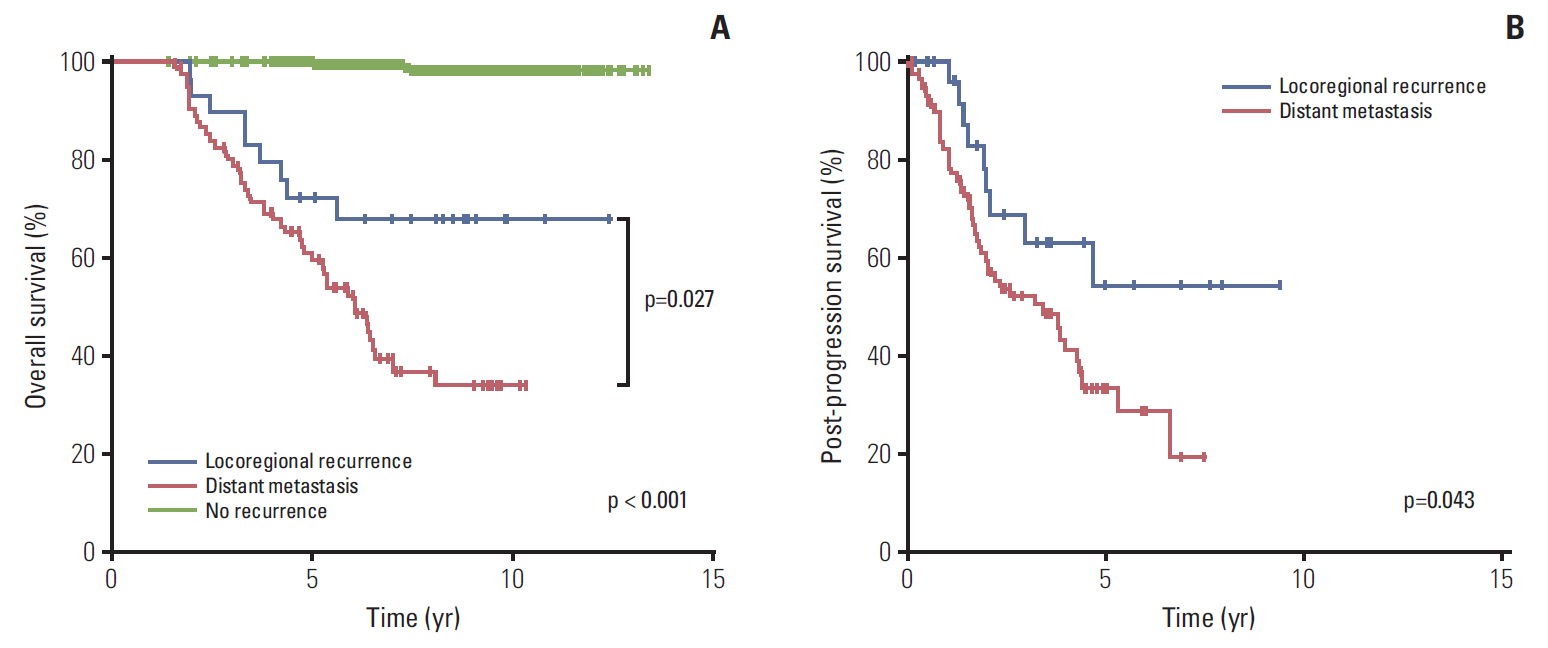
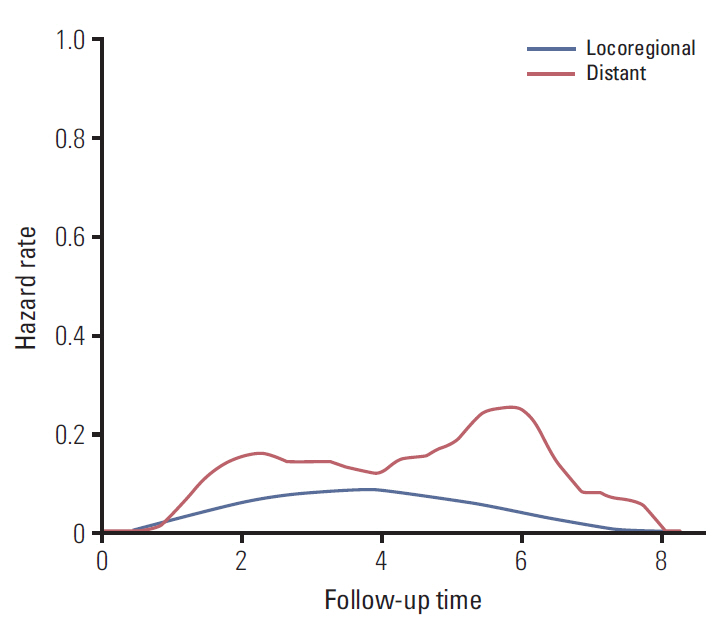
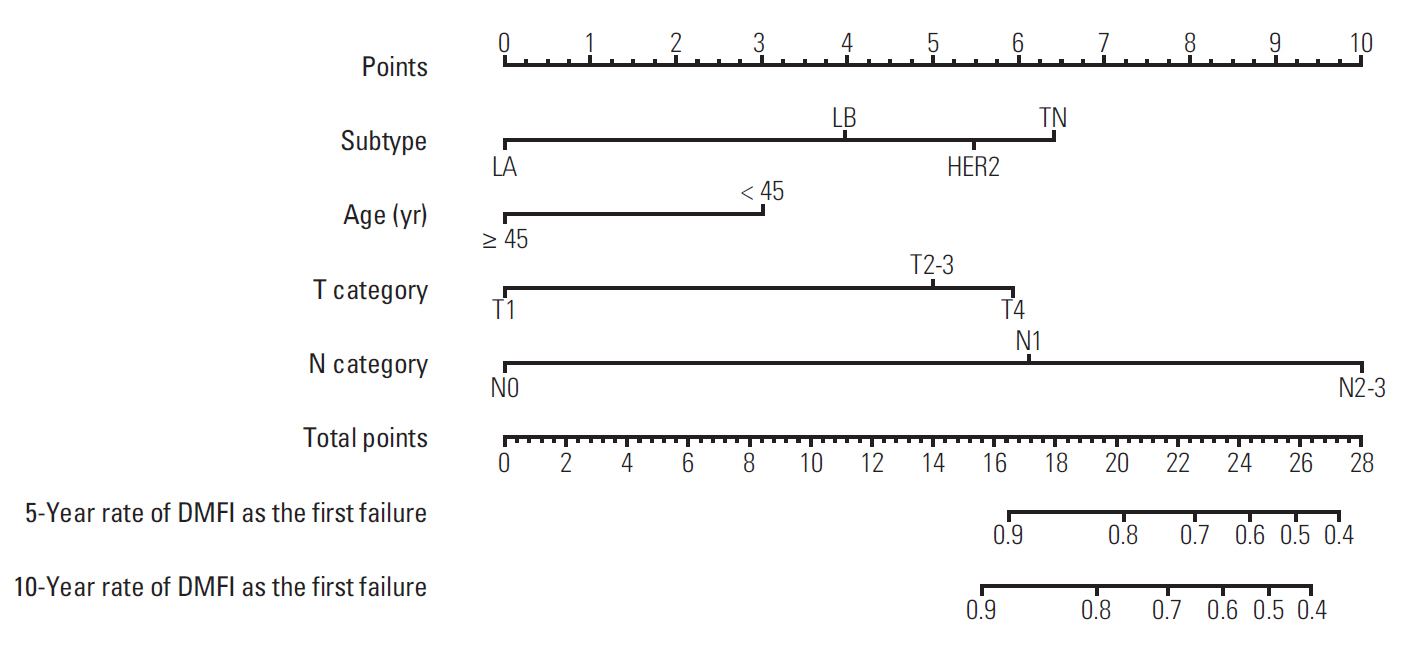
The 7-year overall survival and 5-year post-progression survival of locoregional versus distant recurrence groups were 67.6% versus 39.1% (p=0.027) and 54.2% versus 33.5% (p=0.043), respectively. Patients who developed distant metastasis showed early and late mortality risk peaks within 3 and after 5 years of follow-up, respectively, but a broad and low risk increment was observed in other patients with locoregional relapse. In multivariate analysis of distant metastasis-free interval, age (≥ 45 years vs. < 45 years), molecular subtypes (luminal A vs. luminal B, human epidermal growth receptor 2, and triple negative), T category (T1 vs. T2-3 and T4), and N category (N0 vs. N1 and N2-3) were independently associated (p < 0.05 for all). Regarding the significant factors, a well-validated nomogram was established (concordance index, 0.812). The risk score level of patients with initial brain failure was higher than those of non-brain sites (p=0.029).
CONCLUSION
The nomogram could be useful for predicting the individual probability of distant recurrence in breast cancer. In high-risk patients based on the risk scores, more aggressive systemic therapy and closer surveillance for metastatic failure should be considered.
MeSH Terms
Figure
Cited by 1 articles
-
Score for the Survival Probability in Metastasis Breast Cancer: A Nomogram-Based Risk Assessment Model
Zhenchong Xiong, Guangzheng Deng, Xinjian Huang, Xing Li, Xinhua Xie, Jin Wang, Zeyu Shuang, Xi Wang
Cancer Res Treat. 2018;50(4):1260-1269. doi: 10.4143/crt.2017.443.
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30.
Article2. National Comprehensive Cancer Network. Breast cancer version 2.2017 [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;c2017 [cited 2017 Oct 23]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.3. National Institute for Health and Care Excellence. Adjuvant therapy for early and locally advanced breast cancer [Internet]. London: National Institute for Health and Care Excellence;c2017 [cited 2017 Oct 23]. Available from: http://pathways.nice.org.uk/pathways/early-and-locally-advanced-breast-cancer.4. Wu SG, Li H, Tang LY, Sun JY, Zhang WW, Li FY, et al. The effect of distant metastases sites on survival in de novo stage-IV breast cancer: a SEER database analysis. Tumour Biol. 2017; 39:1010428317705082.
Article5. Wu Q, Li J, Zhu S, Wu J, Chen C, Liu Q, et al. Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget. 2017; 8:27990–6.
Article6. Weigelt B, Peterse JL, van 't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005; 5:591–602.
Article7. Le MG, Arriagada R, Spielmann M, Guinebretiere JM, Rochard F. Prognostic factors for death after an isolated local recurrence in patients with early-stage breast carcinoma. Cancer. 2002; 94:2813–20.
Article8. Lim YJ, Lee SW, Choi N, Kwon J, Eom KY, Kang E, et al. Failure patterns according to molecular subtype in patients with invasive breast cancer following postoperative adjuvant radiotherapy: long-term outcomes in contemporary clinical practice. Breast Cancer Res Treat. 2017; 163:555–63.
Article9. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013; 24:2206–23.10. Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974; 19:716–23.
Article11. Cheng YC, Ueno NT. Improvement of survival and prospect of cure in patients with metastatic breast cancer. Breast Cancer. 2012; 19:191–9.
Article12. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015; 16:e173–80.
Article13. Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006; 24:5652–7.
Article14. Laurberg T, Lyngholm CD, Christiansen P, Alsner J, Overgaard J. Long-term age-dependent failure pattern after breastconserving therapy or mastectomy among Danish lymphnode-negative breast cancer patients. Radiother Oncol. 2016; 120:98–106.
Article15. Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008; 26:3324–30.
Article16. Partridge AH, Hughes ME, Warner ET, Ottesen RA, Wong YN, Edge SB, et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. 2016; 34:3308–14.
Article17. van de Water W, Markopoulos C, van de Velde CJ, Seynaeve C, Hasenburg A, Rea D, et al. Association between age at diagnosis and disease-specific mortality among postmenopausal women with hormone receptor-positive breast cancer. JAMA. 2012; 307:590–7.
Article18. Purushotham A, Shamil E, Cariati M, Agbaje O, Muhidin A, Gillett C, et al. Age at diagnosis and distant metastasis in breast cancer: a surprising inverse relationship. Eur J Cancer. 2014; 50:1697–705.19. Myers CE, Mirza NN, Lustgarten J. Immunity, cancer and aging: lessons from mouse models. Aging Dis. 2011; 2:512–23.20. Labat-Robert J, Robert L. The effect of cell-matrix interactions and aging on the malignant process. Adv Cancer Res. 2007; 98:221–59.21. Hancke K, Denkinger MD, Konig J, Kurzeder C, Wockel A, Herr D, et al. Standard treatment of female patients with breast cancer decreases substantially for women aged 70 years and older: a German clinical cohort study. Ann Oncol. 2010; 21:748–53.
Article22. Arriagada R, Rutqvist LE, Johansson H, Kramar A, Rotstein S. Predicting distant dissemination in patients with early breast cancer. Acta Oncol. 2008; 47:1113–21.
Article23. Ali HR, Rueda OM, Chin SF, Curtis C, Dunning MJ, Aparicio SA, et al. Genome-driven integrated classification of breast cancer validated in over 7,500 samples. Genome Biol. 2014; 15:431.
Article24. Ciriello G, Sinha R, Hoadley KA, Jacobsen AS, Reva B, Perou CM, et al. The molecular diversity of Luminal A breast tumors. Breast Cancer Res Treat. 2013; 141:409–20.
Article25. Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004; 22:3608–17.
Article26. Niwinska A, Murawska M, Pogoda K. Breast cancer brain metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT). Ann Oncol. 2010; 21:942–8.27. Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010; 28:3271–7.
Article28. Almendro V, Kim HJ, Cheng YK, Gonen M, Itzkovitz S, Argani P, et al. Genetic and phenotypic diversity in breast tumor metastases. Cancer Res. 2014; 74:1338–48.
Article29. Fasching PA, Pharoah PD, Cox A, Nevanlinna H, Bojesen SE, Karn T, et al. The role of genetic breast cancer susceptibility variants as prognostic factors. Hum Mol Genet. 2012; 21:3926–39.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Adjuvant Hormonal Therapy on the Development of Pulmonary Fibrosis after Postoperative Radiotherapy for Breast Cancer
- Recent Trend of the Postoperative Adjuvant Therapy in Endometrial Cancer
- Adjuvant therapy in cervical cancer patients with high risk factors
- Radiotherapy for Breast Cancer
- Nomogram for Predicting Survival for Oral Squamous Cell Carcinoma