Clin Nutr Res.
2018 Oct;7(4):276-290. 10.7762/cnr.2018.7.4.276.
Effects of Maternal and Post-Weaning High-Fat Diet on Leptin Resistance and Hypothalamic Appetite Genes in Sprague Dawley Rat Offspring
- Affiliations
-
- 1Department of Home Economics, College of Education, Kyungnam University, Changwon 51767, Korea. jschoi@kyungnam.ac.kr
- KMID: 2424303
- DOI: http://doi.org/10.7762/cnr.2018.7.4.276
Abstract
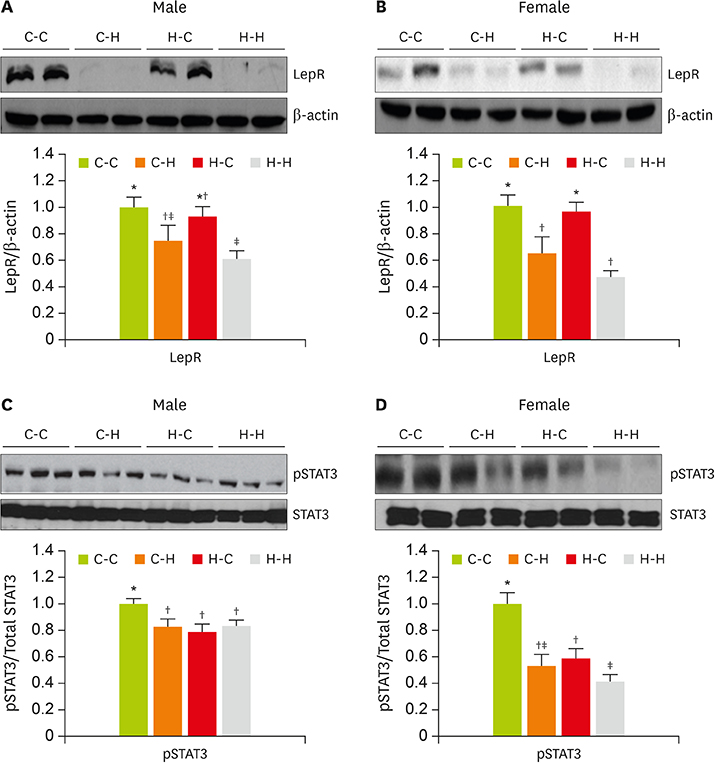
- The defective satiation signaling may contribute to the etiology of obesity. We investigated how dietary modification during maternal (pregnancy and lactation) and post-weaning affects obesity, insulin resistance (IR) and hypothalamic appetite responses in offspring in adulthood. Pregnant female SD rats were randomly allocated to either maternal high-fat diet (43% energy from fat) or control diet (12% energy from fat) until the end of suckling. After weaning for additional 4 weeks, half of the offsprings were continuously fed the same diet as the dam (C-C and H-H groups); the remainder received the counterpart diet (C-H and H-C groups). The long-term high-fat diet during maternal and post-weaning period (H-H group) led to susceptibility to obesity and IR through the significant increases of hypothalamic orexigenic genes compared to the maternal and post-weaning control diet group (C-C group). In contrast, the hypothalamic expression levels of anorexigenic genes, apolipoprotein E, leptin receptor, and activated signal transducer and activator of transcription protein 3 were significantly lower in H-H group with elevations in circulating insulin and leptin and body fat mass. However, dietary changes after weaning (H-C and C-H groups) partially modified these conditions. These results suggest that maternal and post-weaning diet conditions can potentially disrupt hypothalamic neuronal signal irrelevantly, which is essential for leptin's regulation of energy homeostasis and induce the risk of offspring to future metabolic disorders.
Keyword
MeSH Terms
Figure
Reference
-
1. Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol. 2007; 92:287–298.
Article2. Sullivan EL, Nousen EK, Chamlou KA. Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiol Behav. 2014; 123:236–242.
Article3. McMillen IC, Edwards LJ, Duffield J, Muhlhausler BS. Regulation of leptin synthesis and secretion before birth: implications for the early programming of adult obesity. Reproduction. 2006; 131:415–427.
Article4. Gorski JN, Dunn-Meynell AA, Hartman TG, Levin BE. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. Am J Physiol Regul Integr Comp Physiol. 2006; 291:R768–R778.
Article5. Ikenasio-Thorpe BA, Breier BH, Vickers MH, Fraser M. Prenatal influences on susceptibility to diet-induced obesity are mediated by altered neuroendocrine gene expression. J Endocrinol. 2007; 193:31–37.
Article6. McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005; 85:571–633.
Article7. Parent MB, Darling JN, Henderson YO. Remembering to eat: hippocampal regulation of meal onset. Am J Physiol Regul Integr Comp Physiol. 2014; 306:R701–R713.
Article8. Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Häring HU. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev. 2016; 96:1169–1209.
Article9. Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999; 20:68–100.
Article10. Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005; 493:63–71.
Article11. Grattan DR, Ladyman SR, Augustine RA. Hormonal induction of leptin resistance during pregnancy. Physiol Behav. 2007; 91:366–374.
Article12. Palou M, Sánchez J, Rodríguez AM, Priego T, Picó C, Palou A. Induction of NPY/AgRP orexigenic peptide expression in rat hypothalamus is an early event in fasting: relationship with circulating leptin, insulin and glucose. Cell Physiol Biochem. 2009; 23:115–124.
Article13. Shen L, Tso P, Wang DQ, Woods SC, Davidson WS, Sakai R, Liu M. Up-regulation of apolipoprotein E by leptin in the hypothalamus of mice and rats. Physiol Behav. 2009; 98:223–228.
Article14. Shen L, Tso P, Woods SC, Clegg DJ, Barber KL, Carey K, Liu M. Brain apolipoprotein E: an important regulator of food intake in rats. Diabetes. 2008; 57:2092–2098.
Article15. Mela V, Jimenez S, Freire-Regatillo A, Barrios V, Marco EM, Lopez-Rodriguez AB, Argente J, Viveros MP, Chowen JA. Blockage of neonatal leptin signaling induces changes in the hypothalamus associated with delayed pubertal onset and modifications in neuropeptide expression during adulthood in male rats. Peptides. 2016; 86:63–71.
Article16. Song Y, Park MJ, Paik HY, Joung H. Secular trends in dietary patterns and obesity-related risk factors in Korean adolescents aged 10–19 years. Int J Obes. 2010; 34:48–56.
Article17. Chen H, Simar D, Morris MJ. Hypothalamic neuroendocrine circuitry is programmed by maternal obesity: interaction with postnatal nutritional environment. PLoS One. 2009; 4:e6259.
Article18. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–419.
Article19. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959; 37:911–917.
Article20. Tozuka Y, Wada E, Wada K. Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB J. 2009; 23:1920–1934.
Article21. Bayol SA, Simbi BH, Bertrand JA, Stickland NC. Offspring from mothers fed a ‘junk food’ diet in pregnancy and lactation exhibit exacerbated adiposity that is more pronounced in females. J Physiol. 2008; 586:3219–3230.
Article22. Coll AP, Farooqi IS, O'Rahilly S. The hormonal control of food intake. Cell. 2007; 129:251–262.
Article23. Björnholm M, Münzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjørbaek C, Myers MG Jr. Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007; 117:1354–1360.
Article24. Heldsinger A, Grabauskas G, Song I, Owyang C. Synergistic interaction between leptin and cholecystokinin in the rat nodose ganglia is mediated by PI3K and STAT3 signaling pathways: implications for leptin as a regulator of short term satiety. J Biol Chem. 2011; 286:11707–11715.
Article25. Kwon O, Kim KW, Kim MS. Leptin signalling pathways in hypothalamic neurons. Cell Mol Life Sci. 2016; 73:1457–1477.
Article26. Devaskar SU, Ollesch C, Rajakumar RA, Rajakumar PA. Developmental changes in ob gene expression and circulating leptin peptide concentrations. Biochem Biophys Res Commun. 1997; 238:44–47.
Article27. Håkansson-Ovesjö ML, Collin M, Meister B. Down-regulated STAT3 messenger ribonucleic acid and STAT3 protein in the hypothalamic arcuate nucleus of the obese leptin-deficient (ob/ob) mouse. Endocrinology. 2000; 141:3946–3955.28. Frontini A, Bertolotti P, Tonello C, Valerio A, Nisoli E, Cinti S, Giordano A. Leptin-dependent STAT3 phosphorylation in postnatal mouse hypothalamus. Brain Res. 2008; 1215:105–115.
Article29. Chan ES, Chen C, Cole GM, Wong BS. Differential interaction of apolipoprotein-E isoforms with insulin receptors modulates brain insulin signaling in mutant human amyloid precursor protein transgenic mice. Sci Rep. 2015; 5:13842.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-term effects of pro-opiomelanocortin methylation induced in food-restricted dams on metabolic phenotypes in male rat offspring
- Effects of Wax Guard on Weight, Triglyceride, Leptin and Fat Cell Size in Rats Fed on a High Fat Diet
- Effects of disturbed liver growth and oxidative stress of high-fat diet-fed dams on cholesterol metabolism in offspring mice
- Effects of Maternal Folic Acid Nutritional Status on the Expression of Myelin Basic Protein in the Offspring
- Effects of Soy Protein, its Hydrolysate and Peptide Fraction on Lipid Metabolism and Appetite-Related Hormones in Rats