Ann Surg Treat Res.
2018 Nov;95(5):240-248. 10.4174/astr.2018.95.5.240.
ABT-737 ameliorates docetaxel resistance in triple negative breast cancer cell line
- Affiliations
-
- 1Department of Surgery, Seoul Metropolitan Government - Seoul National University Boramae Medical Center, Seoul, Korea. kiterius@snu.ac.kr
- 2Department of Internal Medicine, Seoul Metropolitan Government - Seoul National University Boramae Medical Center, Seoul, Korea.
- 3Department of Biostatistics, Seoul Metropolitan Government - Seoul National University Boramae Medical Center, Seoul, Korea.
- 4Department of Pathology, Seoul Metropolitan Government - Seoul National University Boramae Medical Center, Seoul, Korea.
- KMID: 2422933
- DOI: http://doi.org/10.4174/astr.2018.95.5.240
Abstract
- PURPOSE
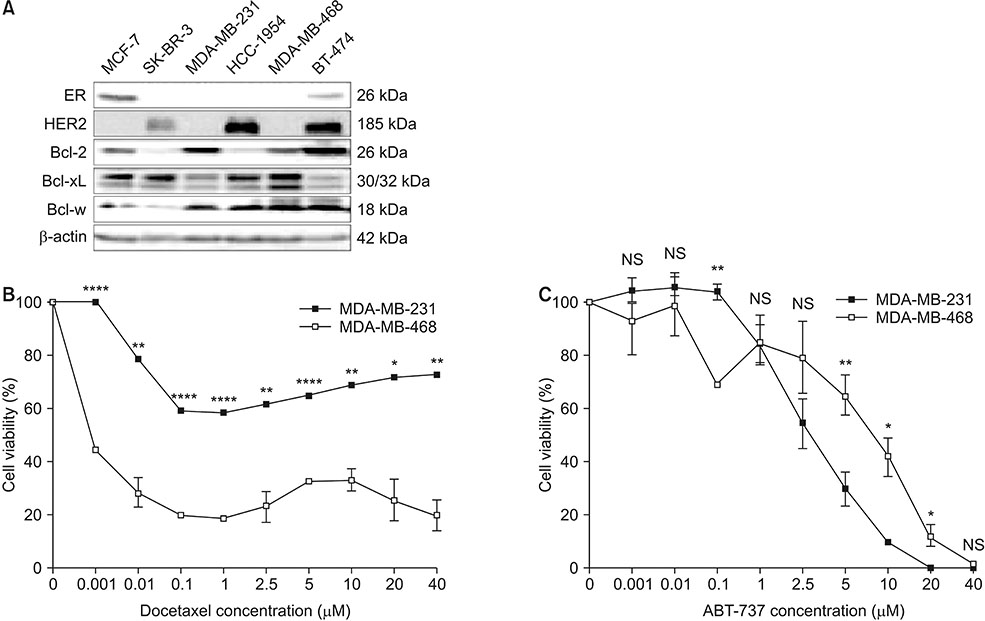
This study aimed to validate the synergistic effect of ABT-737 on docetaxel using MDA-MB-231, a triple negative breast cancer (TNBC) cell line overexpressing B-cell lymphoma-2 (Bcl-2).
METHODS
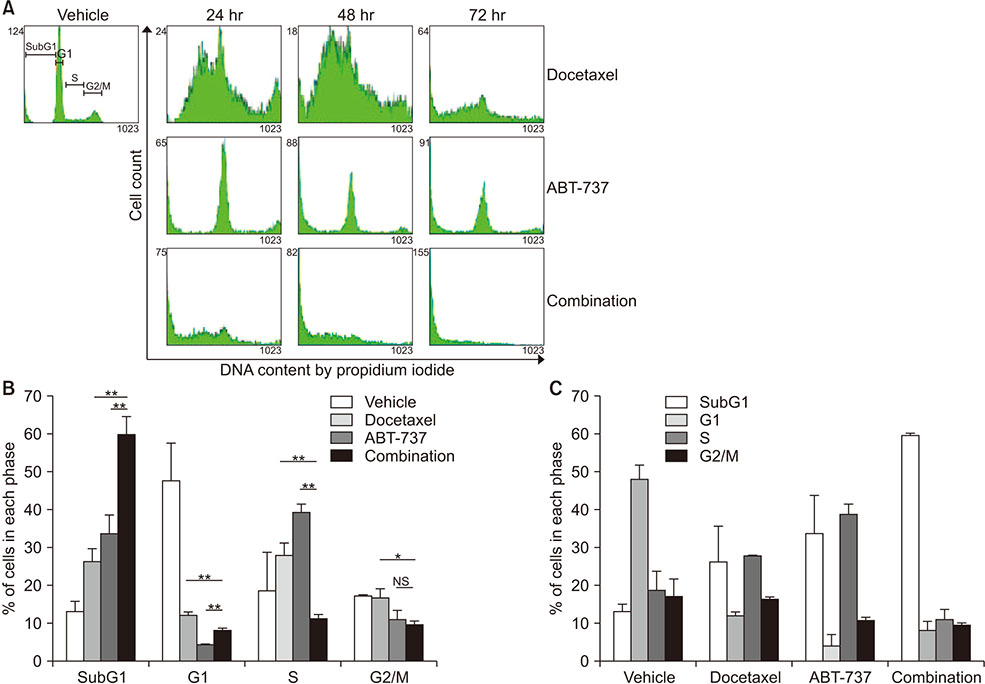
Western blot analysis was performed to assess expression levels of Bcl-2 family proteins and caspase-related molecules. Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cell cycle distribution was determined by flow cytometry analysis. Benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone (z-VAD-fmk) was used for pretreatment to assess the role of caspases.
RESULTS
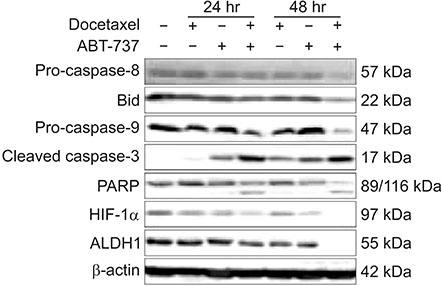
Cell viability of MDA-MB-231 after combination treatment with ABT-737 and docetaxel was significantly lower than that after docetaxel or ABT-737 monotherapy based on MTT assay (both P < 0.001), with a combination index of 0.41. The proportion of sub-G1 population after combination treatment was significantly higher than that after docetaxel or ABT-737 monotherapy (P = 0.001, P = 0.003, respectively). Pretreatment with z-VAD-fmk completely restored cell viability of MDA-MB-231 from apoptotic cell death induced by combination therapy (P = 0.001). Although pro-caspase-8 or Bid did not show significant change in expression level, pro-casepase-9 showed significantly decreased expression after combination treatment. Cleaved caspase-3 showed increased expression while poly (ADP-ribose) polymerase cleavage was induced after combination treatment. However, hypoxia-inducible factor 1-alpha and aldehyde dehydrogenase 1 totally lost their expression after combination treatment.
CONCLUSION
Combination of ABT-737 with docetaxel elicits synergistic therapeutic effect on MDA-MB-231, a TNBC cell line overexpressing Bcl-2, mainly by activating the intrinsic pathway of apoptosis. Therefore, adjunct of ABT-737 to docetaxel might be a new therapeutic option to overcome docetaxel resistance of TNBCs overexpressing Bcl-2.
MeSH Terms
Figure
Reference
-
1. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010; 363:1938–1948.
Article2. Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010; 7:683–692.
Article3. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007; 13(15 Pt 1):4429–4434.
Article4. Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014; 15:49–63.
Article5. Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984; 226:1097–1099.
Article6. Hwang KT, Woo JW, Shin HC, Kim HS, Ahn SK, Moon HG, et al. Prognostic influence of BCL2 expression in breast cancer. Int J Cancer. 2012; 131:E1109–E1119.
Article7. Hwang KT, Han W, Kim J, Moon HG, Oh S, Song YS, et al. Prognostic influence of Bcl2 on molecular subtypes of breast cancer. J Breast Cancer. 2017; 20:54–64.
Article8. Dawson SJ, Makretsov N, Blows FM, Driver KE, Provenzano E, Le Quesne J, et al. BCL2 in breast cancer: a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer. 2010; 103:668–675.
Article9. Callagy GM, Webber MJ, Pharoah PD, Caldas C. Meta-analysis confirms BCL2 is an independent prognostic marker in breast cancer. BMC Cancer. 2008; 8:153.
Article10. Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008; 26:1275–1281.
Article11. Cragg MS, Harris C, Strasser A, Scott CL. Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nat Rev Cancer. 2009; 9:321–326.
Article12. Oakes SR, Vaillant F, Lim E, Lee L, Breslin K, Feleppa F, et al. Sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737. Proc Natl Acad Sci U S A. 2012; 109:2766–2771.
Article13. Panayotopoulou EG, Muller AK, Borries M, Busch H, Hu G, Lev S. Targeting of apoptotic pathways by SMAC or BH3 mimetics distinctly sensitizes paclitaxel-resistant triple negative breast cancer cells. Oncotarget. 2017; 8:45088–45104.
Article14. Kutuk O, Letai A. Alteration of the mitochondrial apoptotic pathway is key to acquired paclitaxel resistance and can be reversed by ABT-737. Cancer Res. 2008; 68:7985–7994.
Article15. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254.
Article16. Witham J, Valenti MR, De-Haven-Brandon AK, Vidot S, Eccles SA, Kaye SB, et al. The Bcl-2/Bcl-XL family inhibitor ABT-737 sensitizes ovarian cancer cells to carboplatin. Clin Cancer Res. 2007; 13:7191–7198.
Article17. Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984; 22:27–55.
Article18. Abramson VG, Lehmann BD, Ballinger TJ, Pietenpol JA. Subtyping of triple-negative breast cancer: implications for therapy. Cancer. 2015; 121:8–16.
Article19. Mayer IA, Abramson VG, Lehmann BD, Pietenpol JA. New strategies for triple-negative breast cancer--deciphering the heterogeneity. Clin Cancer Res. 2014; 20:782–790.
Article20. Rooswinkel RW, van de Kooij B, Verheij M, Borst J. Bcl-2 is a better ABT-737 target than Bcl-xL or Bcl-w and only Noxa overcomes resistance mediated by Mcl-1, Bfl-1, or Bcl-B. Cell Death Dis. 2012; 3:e366.
Article21. Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009; 361:1570–1583.
Article22. Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004; 116:205–219.23. Ajabnoor GM, Crook T, Coley HM. Paclitaxel resistance is associated with switch from apoptotic to autophagic cell death in MCF-7 breast cancer cells. Cell Death Dis. 2012; 3:e260.
Article24. Kepp O, Galluzzi L, Lipinski M, Yuan J, Kroemer G. Cell death assays for drug discovery. Nat Rev Drug Discov. 2011; 10:221–237.
Article25. Ichim G, Tait SW. A fate worse than death: apoptosis as an oncogenic process. Nat Rev Cancer. 2016; 16:539–548.
Article26. Herceg Z, Wang ZQ. Functions of poly (ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat Res. 2001; 477:97–110.27. Samanta D, Gilkes DM, Chaturvedi P, Xiang L, Semenza GL. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc Natl Acad Sci U S A. 2014; 111:E5429–E5438.
Article28. Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev. 2014; 94:1–34.
Article29. Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007; 1:555–567.
Article30. Kida K, Ishikawa T, Yamada A, Shimada K, Narui K, Sugae S, et al. Effect of ALDH1 on prognosis and chemoresistance by breast cancer subtype. Breast Cancer Res Treat. 2016; 156:261–269.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ABT-737, a BH3 Mimetic, Enhances the Therapeutic Effects of Ionizing Radiation in K-ras Mutant Non-Small Cell Lung Cancer Preclinical Model
- Caveolin-1 Modulates Docetaxel-Induced Cell Death in Breast Cancer Cell Subtypes through Different Mechanisms
- Potentiation of the Anticancer Effects by Combining Docetaxel with Ku-0063794 against Triple-Negative Breast Cancer Cells
- Clinicopathologic Characteristics and Prognosis of Early Stage Triple Negative Breast Cancer: Comparison with Non-triple Negative Group
- Bilateral Triple Negative Invasive Ductal Breast Carcinoma in a BRCA1 Mutation Carrier with Discrepant Pathologic Response to Neoadjuvant Chemotherapy