Yonsei Med J.
2018 Nov;59(9):1064-1071. 10.3349/ymj.2018.59.9.1064.
S100A4 Gene is Crucial for Methionine-Choline-Deficient Diet-Induced Non-Alcoholic Fatty Liver Disease in Mice
- Affiliations
-
- 1Department of Infectious Diseases, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, China. ddp_ding@sina.com
- 2Maternal and Child Health-Care Hospital, Shiyan, Hubei, China.
- KMID: 2422491
- DOI: http://doi.org/10.3349/ymj.2018.59.9.1064
Abstract
- PURPOSE
To explore the influence of S100 calcium binding protein A4 (S100A4) knockout (KO) on methionine-choline-deficient (MCD) diet-induced non-alcoholic fatty liver disease (NAFLD) in mice.
MATERIALS AND METHODS
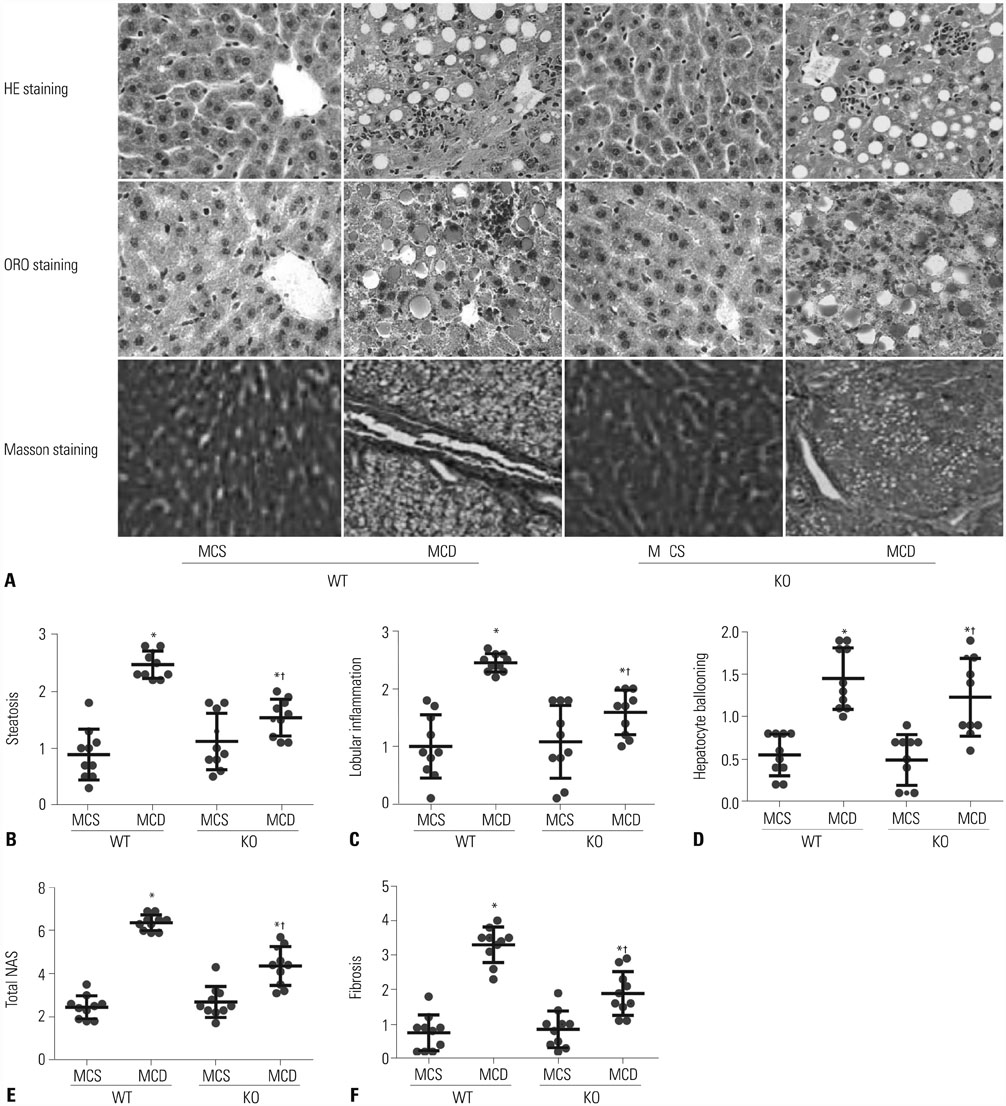
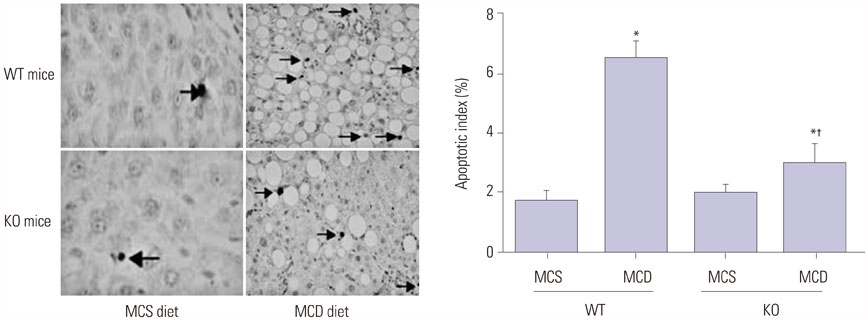
S100A4 KO mice (n=20) and their wild-type (WT) counterparts (n=20) were randomly divided into KO/MCD, Ko/methionine-choline-sufficient (MCS), WT/MCD, and WT/MCS groups. After 8 weeks of feeding, blood lipid and liver function-related indexes were measured. HE, Oil Red O, and Masson stainings were used to observe the changes of liver histopathology. Additionally, expressions of S100A4 and proinflammatory and profibrogenic cytokines were detected by qRT-PCR and Western blot, while hepatocyte apoptosis was revealed by TUNEL staining.
RESULTS
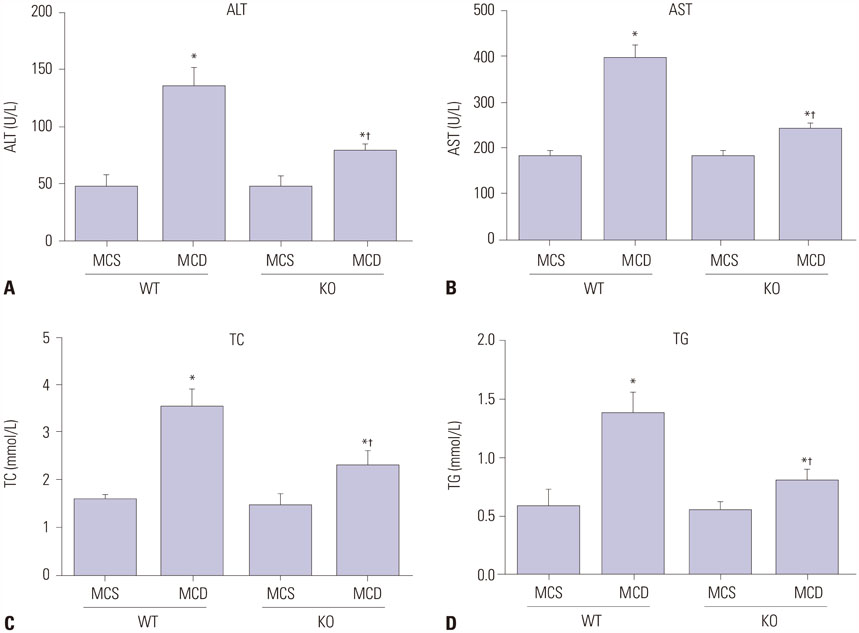
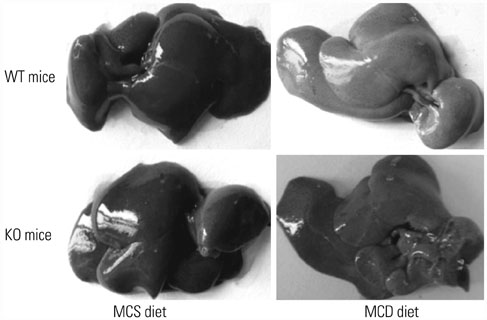
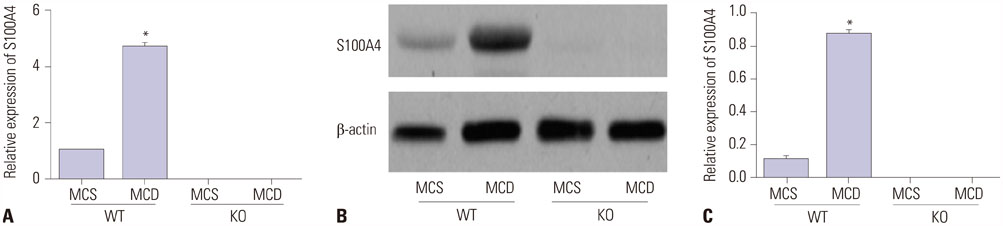
Serum levels of aminotransferase, aspartate aminotransferase, triglyceride, and total cholesterol in mice were increased after 8-week MCD feeding, and hepatocytes performed varying balloon-like changes with increased inflammatory cell infiltration and collagen fibers; however, these effects were improved in mice of KO/MCD group. Meanwhile, total NAFLD activity scores and fibrosis were lower compared to WT+MCD group. Compared to WT/MCS group, S100A4 expression in liver tissue of WT/MCD group was enhanced. The expression of proinflammatory (TNF-α, IL-1β, IL-6) and profibrogenic cytokines (TGF-β1, COL1A1, α-SMA) in MCD-induced NAFLD mice were increased, as well as apoptotic index (AI). For MCD group, the expressions of proinflammatory and profibrogenic cytokines and AI in KO mice were lower than those of WT mice.
CONCLUSION
S100A4 was detected to be upregulated in NAFLD, while S100A4 KO alleviated liver fibrosis and inflammation, in addition to inhibiting hepatocyte apoptosis.
Keyword
MeSH Terms
-
Animals
Apoptosis
Aspartate Aminotransferases
Blotting, Western
Calcium
Carrier Proteins
Cholesterol
Collagen
Cytokines
Fibrosis
Hepatocytes
In Situ Nick-End Labeling
Inflammation
Liver
Liver Cirrhosis
Mice*
Non-alcoholic Fatty Liver Disease*
Triglycerides
Aspartate Aminotransferases
Calcium
Carrier Proteins
Cholesterol
Collagen
Cytokines
Figure
Reference
-
1. Arner P, Petrus P, Esteve D, Boulomié A, Näslund E, Thorell A, et al. Screening of potential adipokines identifies S100A4 as a marker of pernicious adipose tissue and insulin resistance. Int J Obes (Lond). 2018; 01. 30. [Epub]. DOI: 10.1038/s41366-018-0018-0.
Article2. Afonso MB, Rodrigues PM, Simão AL, Castro RE. Circulating microRNAs as potential biomarkers in non-alcoholic fatty liver disease and hepatocellular carcinoma. J Clin Med. 2016; 5:pii: E30.
Article3. Nair S. Nonalcoholic Fatty liver disease from the perspective of an internist. Ochsner J. 2002; 4:92–97.4. Patel A, Harrison SA. Hepatitis C virus infection and nonalcoholic steatohepatitis. Gastroenterol Hepatol (N Y). 2012; 8:305–312.5. Dongiovanni P, Valenti L. Genetics of nonalcoholic fatty liver disease. Metabolism. 2016; 65:1026–1037.
Article6. Saleh A, Kamel L, Ghali A, Ismail A, El Khayat H. Serum levels of astroglial S100-beta and neuron-specific enolase in hepatic encephalopathy patients. East Mediterr Health J. 2007; 13:1114–1123.
Article7. Dempsey BR, Rintala-Dempsey AC, Shaw GS. S100 proteins. In : Choi S, editor. Encyclopedia of signaling molecules. New York (NY): Springer;2012. p. 1711–1717.8. Mukai K, Miyagi T, Nishio K, Yokoyama Y, Yoshioka T, Saito Y, et al. S100A8 production in CXCR2-expressing CD11b+Gr-1high cells aggravates hepatitis in mice fed a high-fat and high-cholesterol diet. J Immunol. 2016; 196:395–406.
Article9. Liu X, Wang Y, Ming Y, Song Y, Zhang J, Chen X, et al. S100A9: a potential biomarker for the progression of non-alcoholic fatty liver disease and the diagnosis of non-alcoholic steatohepatitis. PLoS One. 2015; 10:e0127352.
Article10. Malashkevich VN, Dulyaninova NG, Ramagopal UA, Liriano MA, Varney KM, Knight D, et al. Phenothiazines inhibit S100A4 function by inducing protein oligomerization. Proc Natl Acad Sci U S A. 2010; 107:8605–8610.
Article11. Hou S, Tian T, Qi D, Sun K, Yuan Q, Wang Z, et al. S100A4 promotes lung tumor development through β-catenin pathway-mediated autophagy inhibition. Cell Death Dis. 2018; 9:277.
Article12. Oslejsková L, Grigorian M, Gay S, Neidhart M, Senolt L. The metastasis associated protein S100A4: a potential novel link to inflammation and consequent aggressive behaviour of rheumatoid arthritis synovial fibroblasts. Ann Rheum Dis. 2008; 67:1499–1504.
Article13. Cerezo LA, Kuncová K, Mann H, Tomcík M, Zámecník J, Lukanidin E, et al. The metastasis promoting protein S100A4 is increased in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2011; 50:1766–1772.
Article14. Louka ML, Ramzy MM. Involvement of fibroblast-specific protein 1 (S100A4) and matrix metalloproteinase-13 (MMP-13) in CCl4-induced reversible liver fibrosis. Gene. 2016; 579:29–33.
Article15. Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013; 10:627–636.
Article16. Okubo H, Kushiyama A, Sakoda H, Nakatsu Y, Iizuka M, Taki N, et al. Involvement of resistin-like molecule β in the development of methionine-choline deficient diet-induced non-alcoholic steatohepatitis in mice. Sci Rep. 2016; 6:20157.
Article17. Rizki G, Arnaboldi L, Gabrielli B, Yan J, Lee GS, Ng RK, et al. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J Lipid Res. 2006; 47:2280–2290.
Article18. Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res. 2008; 49:1068–1076.
Article19. Wang Y, Li J, Zhuge L, Su D, Yang M, Tao S, et al. Comparison between the efficacies of curcumin and puerarin in C57BL/6 mice with steatohepatitis induced by a methionine- and choline-deficient diet. Exp Ther Med. 2014; 7:663–668.
Article20. Kim SB, Kang OH, Lee YS, Han SH, Ahn YS, Cha SW, et al. Hepatoprotective effect and synergism of bisdemethoycurcumin against MCD diet-induced nonalcoholic fatty liver disease in mice. PLoS One. 2016; 11:e0147745.
Article21. National Research Council. Guide for the care and use of laboratory animals. 8th ed. Washington (DC): National Academies Press;2011.22. EL Naaman C, Grum-Schwensen B, Mansouri A, Grigorian M, Santoni-Rugiu E, Hansen T, et al. Cancer predisposition in mice deficient for the metastasis-associated Mts1(S100A4) gene. Oncogene. 2004; 23:3670–3680.
Article23. Alam S, Alam M, Alam SMNE, Chowdhury ZR, Kabir J. Prevalence and predictor of nonalcoholic steatohepatitis (NASH) in nonalcoholic fatty liver disease (NAFLD). J Bangladesh Coll Phys Surg. 2014; 32:71–77.
Article24. García-Galiano D, Sánchez-Garrido MA, Espejo I, Montero JL, Costán G, Marchal T, et al. IL-6 and IGF-1 are independent prognostic factors of liver steatosis and non-alcoholic steatohepatitis in morbidly obese patients. Obes Surg. 2007; 17:493–503.
Article25. Ying LI, Hong ZF. Hypothesis of “two hits” in NAFLD. Med Recapitulate. 2013; 19:594–596.26. Abu El-Asrar AM, Nawaz MI, De Hertogh G, Alam K, Siddiquei MM, Van den Eynde K, et al. S100A4 is upregulated in proliferative diabetic retinopathy and correlates with markers of angiogenesis and fibrogenesis. Mol Vis. 2014; 20:1209–1224.27. Takeuchi M, Takino JI, Sakasai-Sakai A, Takata T, Tsutsumi M. Toxic AGE (TAGE) theory for the pathophysiology of the onset/progression of NAFLD and ALD. Nutrients. 2017; 9:pii: E634.
Article28. Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010; 52:1836–1846.
Article29. Berlanga A, Guiu-Jurado E, Porras JA, Auguet T. Molecular pathways in non-alcoholic fatty liver disease. Clin Exp Gastroenterol. 2014; 7:221–239.30. Klingelhöfer J, Senolt L, Baslund B, Nielsen GH, Skibshøj I, Pavelka K, et al. Up-regulation of metastasis-promoting S100A4 (Mts-1) in rheumatoid arthritis: putative involvement in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2007; 56:779–789.
Article31. Tilg H, Moschen AR, Szabo G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2016; 64:955–965.
Article32. Sharifnia T, Antoun J, Verriere TG, Suarez G, Wattacheril J, Wilson KT, et al. Hepatic TLR4 signaling in obese NAFLD. Am J Physiol Gastrointest Liver Physiol. 2015; 309:G270–G278.
Article33. Chen L, Li J, Zhang J, Dai C, Liu X, Wang J, et al. S100A4 promotes liver fibrosis via activation of hepatic stellate cells. J Hepatol. 2015; 62:156–164.
Article34. Yan LB, Zhang QB, Zhu X, He M, Tang H. Serum S100 calcium binding protein A4 improves the diagnostic accuracy of transient elastography for assessing liver fibrosis in hepatitis B. Clin Res Hepatol Gastroenterol. 2018; 42:64–71.
Article35. Schneider M, Hansen JL, Sheikh SP. S100A4: a common mediator of epithelial-mesenchymal transition, fibrosis and regeneration in diseases? J Mol Med (Berl). 2008; 86:507–522.
Article36. Syn WK, Jung Y, Omenetti A, Abdelmalek M, Guy CD, Yang L, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009; 137:1478–1488.
Article37. Maher JJ. Pathogenesis of NAFLD and NASH. In : Chalasani N, Szabo G, editors. Alcoholic and non-alcoholic fatty liver disease. Cham: Springer;2016. p. 71–101.38. Mazzucchelli L. Protein S100A4: too long overlooked by pathologists? Am J Pathol. 2002; 160:7–13.
Article39. Tomita K, Teratani T, Suzuki T, Oshikawa T, Yokoyama H, Shimamura K, et al. p53/p66Shc-mediated signaling contributes to the progression of non-alcoholic steatohepatitis in humans and mice. J Hepatol. 2012; 57:837–843.
Article40. Orre LM, Panizza E, Kaminskyy VO, Vernet E, Gräslund T, Zhivotovsky B, et al. S100A4 interacts with p53 in the nucleus and promotes p53 degradation. Oncogene. 2013; 32:5531–5540.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Downregulation of GNAI3 Promotes the Pathogenesis of Methionine/Choline-Deficient Diet-Induced Nonalcoholic Fatty Liver Disease
- Comparative study of fatty liver induced by methionine and choline-deficiency in C57BL/6N mice originating from three different sources
- Dietary Oleate Has Beneficial Effects on Every Step of Non-Alcoholic Fatty Liver Disease Progression in a Methionine- and Choline-Deficient Diet-Fed Animal Model
- Comparative study of liver injury induced by high-fat methionine- and choline-deficient diet in ICR mice originating from three different sources
- Feasibility and Stability of Liver Biopsy before Treatment for Preclinical Nonalcoholic Fatty Liver Studies