J Pathol Transl Med.
2018 Sep;52(5):314-322. 10.4132/jptm.2018.07.17.
An Immunohistochemical and Polarizing Microscopic Study of the Tumor Microenvironment in Varying Grades of Oral Squamous Cell Carcinoma
- Affiliations
-
- 1Department of Pathology, Jawahar Lal Nehru Medical College, Aligarh, India.
- 2Department of Oral Pathology and Microbiology, Sardar Patel Post Graduate Institute of Dental & Medical Sciences, Lucknow, India. safiasidd4@gmail.com
- 3Department of Community Medicine, Jawahar Lal Nehru Medical College, AMU, Aligarh, India.
- 4Department of Oral Pathology and Microbiology, King George Medical University, Lucknow, India.
- KMID: 2422098
- DOI: http://doi.org/10.4132/jptm.2018.07.17
Abstract
- BACKGROUND
Invasion of epithelial cells into the connective tissue brings about massive morphological and architectural changes in the underlying stroma. Myofibroblasts reorganize the stroma to facilitate the movement of tumor cells leading to metastasis. The aim of this study was to determine the number and pattern of distribution of myofibroblasts and the qualitative and quantitative change that they cause in the collagen present in the stroma in various grades of oral squamous cell carcinoma (OSCC).
METHODS
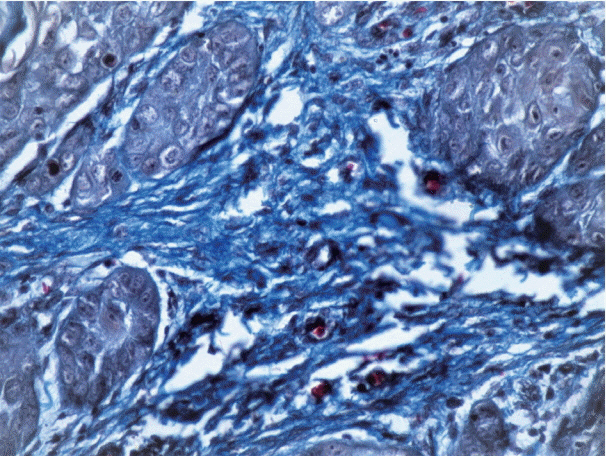
The study was divided into two groups with group I (test group, 65 cases) consisting of 29 cases of well-differentiated squamous cell carcinoma, 25 moderately differentiated SCC, and 11 poorly differentiated SCC, and group II (control group) consisting of 11 cases of normal mucosa. Sections from each sample were stained with anti-α-smooth muscle actin (α-SMA) antibodies, hematoxylin and eosin, and Picrosirius red. Several additional sections from each grade of OSCC were stained with Masson's trichrome to observe the changes in collagen. For the statistical analysis, Fisher's exact test, Tukey's post hoc honest significant difference test, ANOVA, and the chi-square test were used, and p < .05 was considered statistically significant.
RESULTS
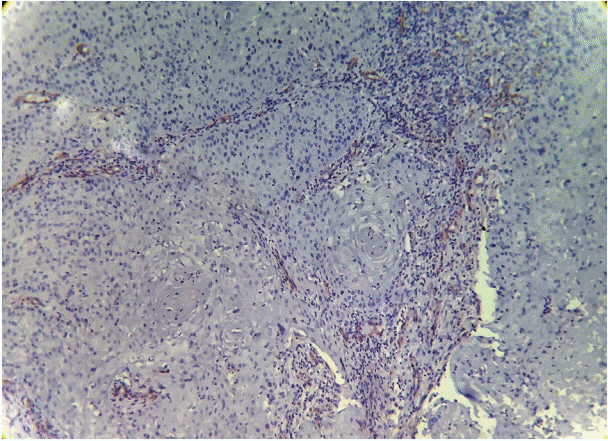
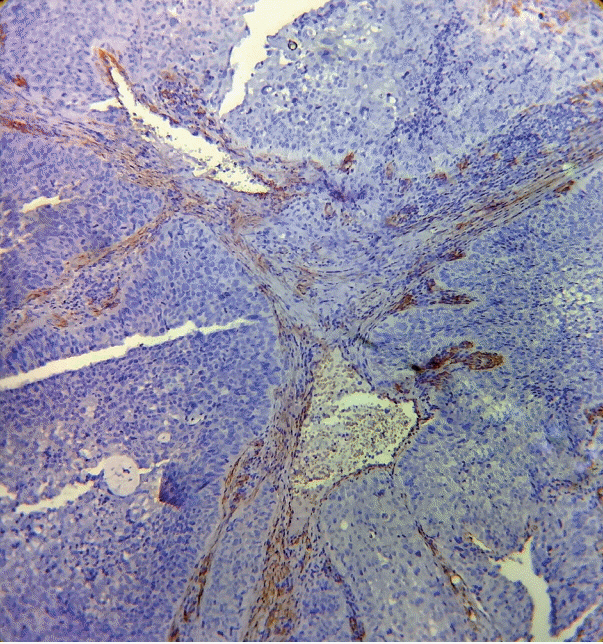
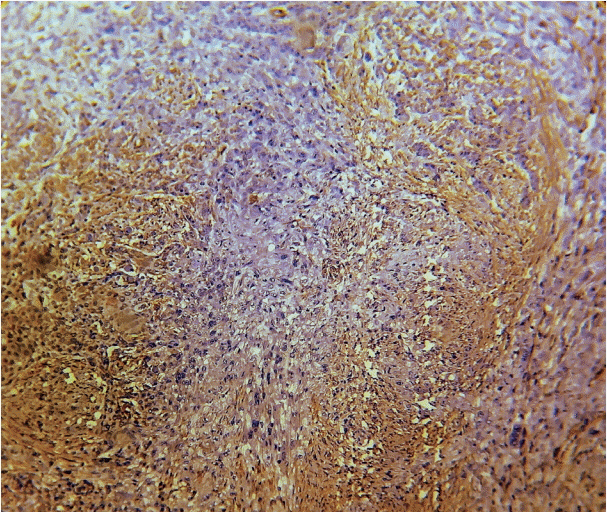
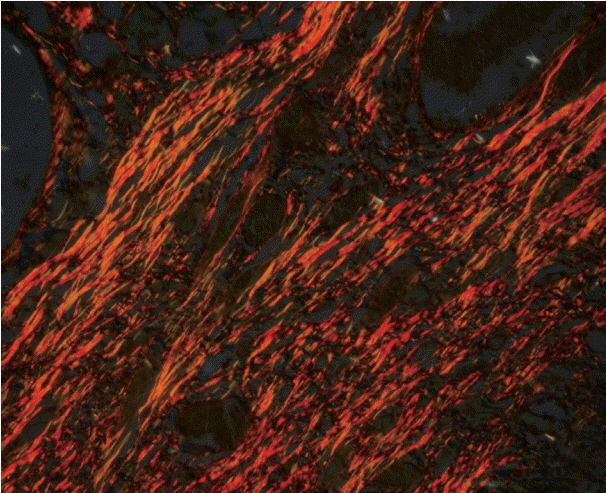
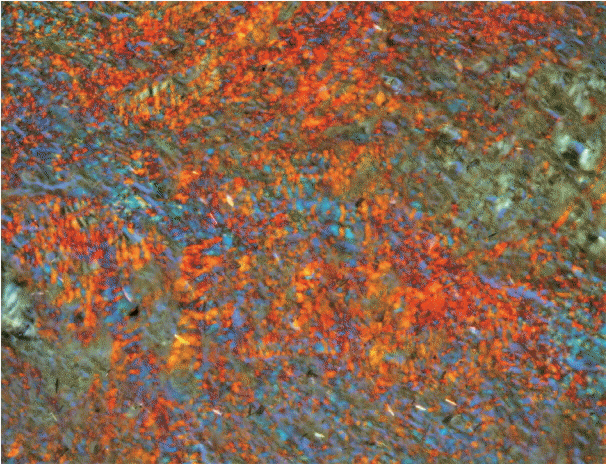
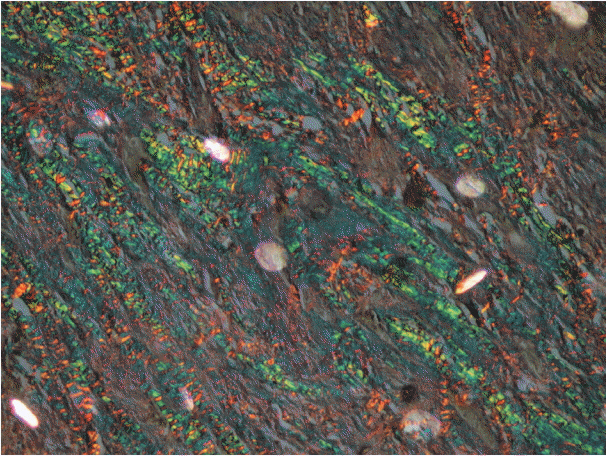
As the tumor stage progressed, an increase in the intensity α-SMA expression was seen, and the network pattern dominated in more dedifferentiated carcinomas. The collagen fibers became thin, loosely packed, and haphazardly aligned with progressing cancer. Additionally, the mean area fraction decreased, and the fibers attained a greenish yellow hue and a weak birefringence when observed using polarizing light microscopy.
CONCLUSIONS
Myofibroblasts bring about numerous changes in collagen. As cancer progresses, there isincrease in pathological collagen,which enhances the movement of cells within the stroma.
Keyword
MeSH Terms
Figure
Reference
-
1. Khandekar SP, Bagdey PS, Tiwari RR. Oral cancer and some epidemiological factors: a hospital based study. Indian J Commun Med. 2006; 31:157–9.2. Coelho KR. Challenges of the oral cancer burden in India. J Cancer Epidemiol. 2012; 2012:701932.
Article3. Aparna V, Charu S. Evaluation of collagen in different grades of oral squamous cell carcinoma by using the picrosirius red stain: a histochemical study. J Clin Diagn Res. 2010; 4:3444–9.4. Shrestha P, Sakamoto F, Takagi H, Yamada T, Mori M. Enhanced tenascin immunoreactivity in leukoplakia and squamous cell carcinoma of the oral cavity: an immunohistochemical study. Eur J Cancer B Oral Oncol. 1994; 30B:132–7.
Article5. van den Hooff A. Stromal involvement in malignant growth. Adv Cancer Res. 1988; 50:159–96.
Article6. Arun Gopinathan P, Kokila G, Jyothi M, Ananjan C, Pradeep L, Humaira Nazir S. Study of collagen birefringence in different grades of oral squamous cell carcinoma using picrosirius red and polarized light microscopy. Scientifica (Cairo). 2015; 2015:802980.
Article7. Kardam P, Mehendiratta M, Rehani S, Kumra M, Sahay K, Jain K. Stromal fibers in oral squamous cell carcinoma: a possible new prognostic indicator? J Oral Maxillofac Pathol. 2016; 20:405–12.
Article8. Nakayama H, Enzan H, Miyazaki E, Naruse K, Kiyoku H, Hiroi M. The role of myofibroblasts at the tumor border of invasive colorectal adenocarcinomas. Jpn J Clin Oncol. 1998; 28:615–20.
Article9. Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999; 277:C1–9.
Article10. Rich L, Whittaker P. Collagen and picrosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci. 2005; 22:97–104.11. Bancroft JD, Gamble M. Theory and practice of histological techniques. 6th ed. London: Churchill Livingstone;2008.12. Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001; 166:2472–83.
Article13. Vered M, Allon I, Buchner A, Dayan D. Stromal myofibroblasts accompany modifications in the epithelial phenotype of tongue dysplastic and malignant lesions. Cancer Microenviron. 2009; 2:49–57.
Article14. Singh HP, Shetty DC, Wadhwan V, Aggarwal P. A quantitative and qualitative comparative analysis of collagen fibers to determine the role of connective tissue stroma on biological behavior of odontogenic cysts: a histochemical study. Natl J Maxillofac Surg. 2012; 3:15–20.
Article15. Montes GS, Junqueira LC. The use of the Picrosirius-polarization method for the study of the biopathology of collagen. Mem Inst Oswaldo Cruz. 1991; 86 Suppl 3:1–11.
Article16. Kalele KK, Managoli NA, Roopa NM, Kulkarni M, Bagul N, Kheur S. Assessment of collagen fibre nature, spatial distribution, hue and its correlation with invasion and metastasis in oral squamous cell carcinoma and surgical margins using picrosirius red and polarized microscope. J Dent Res Rev. 2014; 1:14–7.17. Alrani D, Niranjan KC, Acharya S, Hallikeri K. Histochemical analysis of collagen reorganization at the tumor-stromal interface in oral squamous cell carcinoma: a polarizing microscopic study. Austin J Dent. 2016; 3:1–7.18. Martins GB, Reis SR, Silva TM. Collagen type I expression in squamous cell carcinoma of the oral cavity. Pesqui Odontol Bras. 2003; 17:82–8.19. Schantz SP, Yu GP. Head and neck cancer incidence trends in young Americans, 1973-1997, with a special analysis for tongue cancer. Arch Otolaryngol Head Neck Surg. 2002; 128:268–74.
Article20. Llewellyn CD, Johnson NW, Warnakulasuriya KA. Risk factors for squamous cell carcinoma of the oral cavity in young people: a comprehensive literature review. Oral Oncol. 2001; 37:401–18.
Article21. Warnakulasuriya S. Living with oral cancer: epidemiology with particular reference to prevalence and life-style changes that influence survival. Oral Oncol. 2010; 46:407–10.
Article22. Silverman S Jr, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation: a follow-up study of 257 patients. Cancer. 1984; 53:563–8.
Article23. Shimasaki N, Kuroda N, Miyazaki E, et al. The distribution pattern of myofibroblasts in the stroma of human bladder carcinoma depends on their invasiveness. Histol Histopathol. 2006; 21:349–53.24. Chauhan H, Abraham A, Phillips JR, Pringle JH, Walker RA, Jones JL. There is more than one kind of myofibroblast: analysis of CD34 expression in benign, in situ, and invasive breast lesions. J Clin Pathol. 2003; 56:271–6.
Article25. Seifi S, Shafaei S, Shafigh E, Sahabi SM, Ghasemi H. Myofibroblast stromal presence and distribution in squamous epithelial carcinomas, oral dysplasia and hyperkeratosis. Asian Pac J Cancer Prev. 2010; 11:359–64.26. Kellermann MG, Sobral LM, da Silva SD, et al. Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral Oncol. 2008; 44:509–17.
Article27. Marsh D, Suchak K, Moutasim KA, et al. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol. 2011; 223:470–81.
Article28. Evanko SP, Potter-Perigo S, Petty LJ, Workman GA, Wight TN. Hyaluronan controls the deposition of fibronectin and collagen and modulates TGF-beta1 induction of lung myofibroblasts. Matrix Biol. 2015; 42:74–92.29. Zhou ZH, Ji CD, Xiao HL, Zhao HB, Cui YH, Bian XW. Reorganized collagen in the tumor microenvironment of gastric cancer and its association with prognosis. J Cancer. 2017; 8:1466–76.
Article30. Provenzano PP, Inman DR, Eliceiri KW, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008; 6:11.
Article31. John RE, Murthy S. Morphological analysis of collagen and elastic fibers in oral squamous cell carcinoma using special stains and comparison with Broder's and Bryne's grading systems. Indian J Dent Res. 2016; 27:242–8.
Article32. Yokoyama M. Alterations in stromal reaction during tumour progression in oral mucosa. J Hard Tissue Biol. 2011; 20:23–30.33. Davies KJ. The Complex interaction of matrix metalloproteinases in the migration of cancer cells through breast tissue stroma. Int J Breast Cancer. 2014; 2014:839094.
Article34. Zhang Z, Tao D, Zhang P, et al. Hyaluronan synthase 2 expressed by cancer-associated fibroblasts promotes oral cancer invasion. J Exp Clin Cancer Res. 2016; 35:181.
Article35. Manjunatha BS, Agrawal A, Shah V. Histopathological evaluation of collagen fibres using picrosirius red stain and polarizing microscopy in oral squamous cell carcinoma. J Can Res Ther. 2015; 11:272–6.36. Fang M, Yuan J, Peng C, Li Y. Collagen as a double-edged sword in tumor progression. Tumour Biol. 2014; 35:2871–82.
Article37. Conklin MW, Eickhoff JC, Riching KM, et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011; 178:1221–32.
Article38. Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009; 139:891–906.
Article39. Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006; 4:38.
Article40. Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005; 8:241–54.
Article41. George J, Narang RS, Rao NN. Stromal response in different histological grades of oral squamous cell carcinoma: a histochemical study. Indian J Dent Res. 2012; 23:842.
Article42. Ziober BL, Turner MA, Palefsky JM, Banda MJ, Kramer RH. Type I collagen degradation by invasive oral squamous cell carcinoma. Oral Oncol. 2000; 36:365–72.
Article43. Dayan D, Hiss Y, Hirshberg A, Bubis JJ, Wolman M. Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry. 1989; 93:27–9.
Article44. Hanley CJ, Mellone M, Ford K, et al. Targeting the myofibroblastic cancer-associated fibroblast phenotype through inhibition of NOX4. J Natl Cancer Inst. 2018; 110:109–20.
Article45. Grossman M, Ben-Chetrit N, Zhuravlev A, et al. Tumor cell invasion can be blocked by modulators of collagen fibril alignment that control assembly of the extracellular matrix. Cancer Res. 2016; 76:4249–58.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnostic problem of squamous papilloma and oral mucosa malignancy
- Squamous cell carcinoma arising in an odontogenic cyst
- Determination of pretreatment serum CEA and SCCA in patients with oral squamous cell carcinoma
- The Tumor Immune Microenvironment in Cutaneous Squamous Cell Carcinoma Arising in Organ Transplant Recipients
- IMMUNOHISTOCHEMICAL STUDY OF AURORA-2 KINASE IN THE ORAL SQUAMOUS CELL CARCINOMA