Nat Prod Sci.
2018 Sep;24(3):199-205. 10.20307/nps.2018.24.3.199.
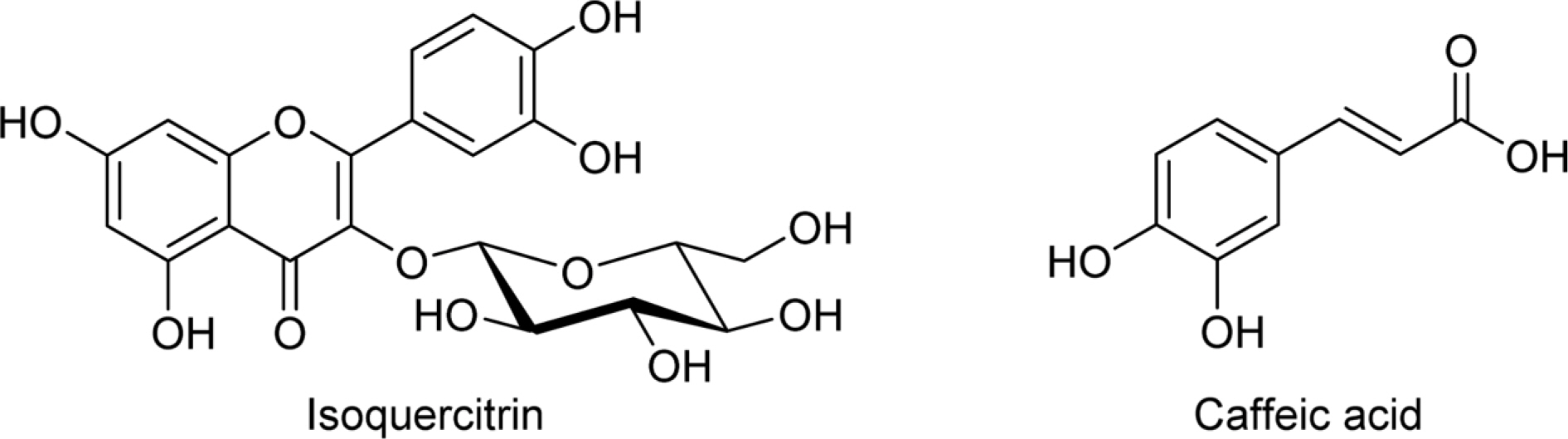
Optimization of Extraction Conditions and Quantitative Analysis of Isoquercitrin and Caffeic Acid from Aster scaber
- Affiliations
-
- 1Department of Integrative Plant Science, Chung-Ang University, Anseong 17546, Republic of Korea. slee@cau.ac.kr
- 2Department of Biology, Soonchunhyang University, Asan 31538, Republic of Korea.
- 3School of Pharmacy, Minzu University of China, Beijing 100081, China.
- 4Department of Food Science and Nutrition, Gwangju University, Gwangju 61743, Republic of Korea. mijachung@gwangju.ac.kr
- KMID: 2422013
- DOI: http://doi.org/10.20307/nps.2018.24.3.199
Abstract
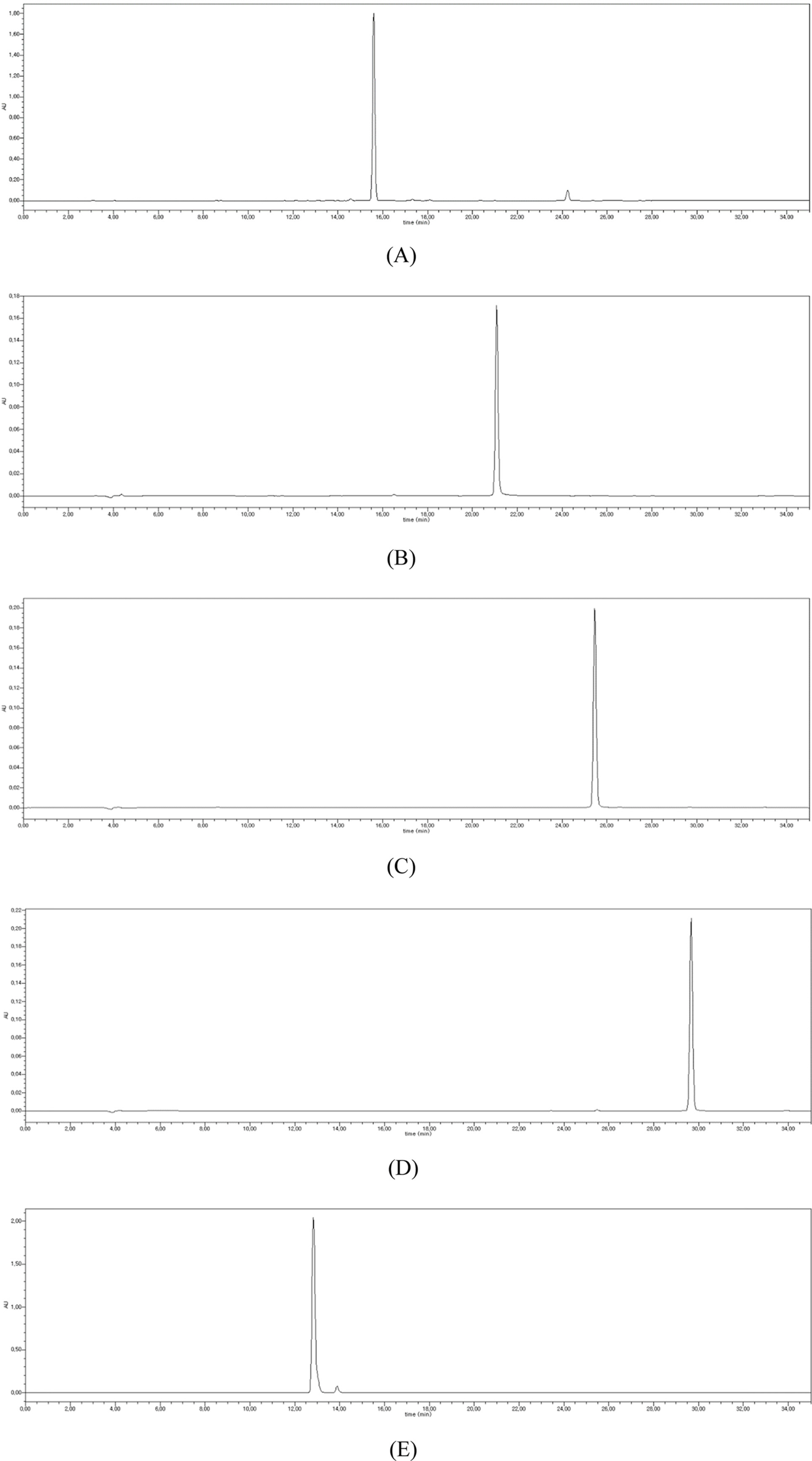
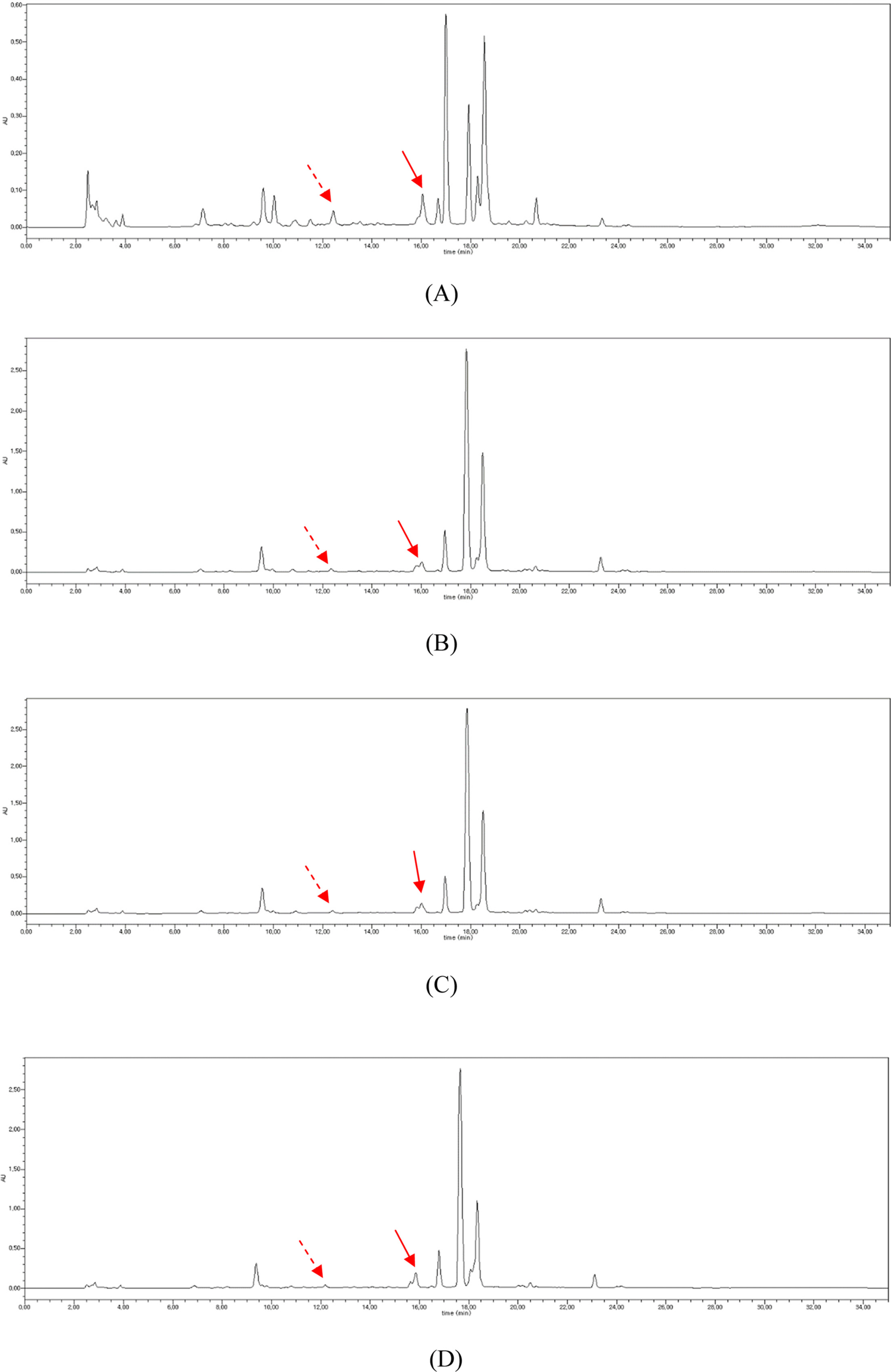
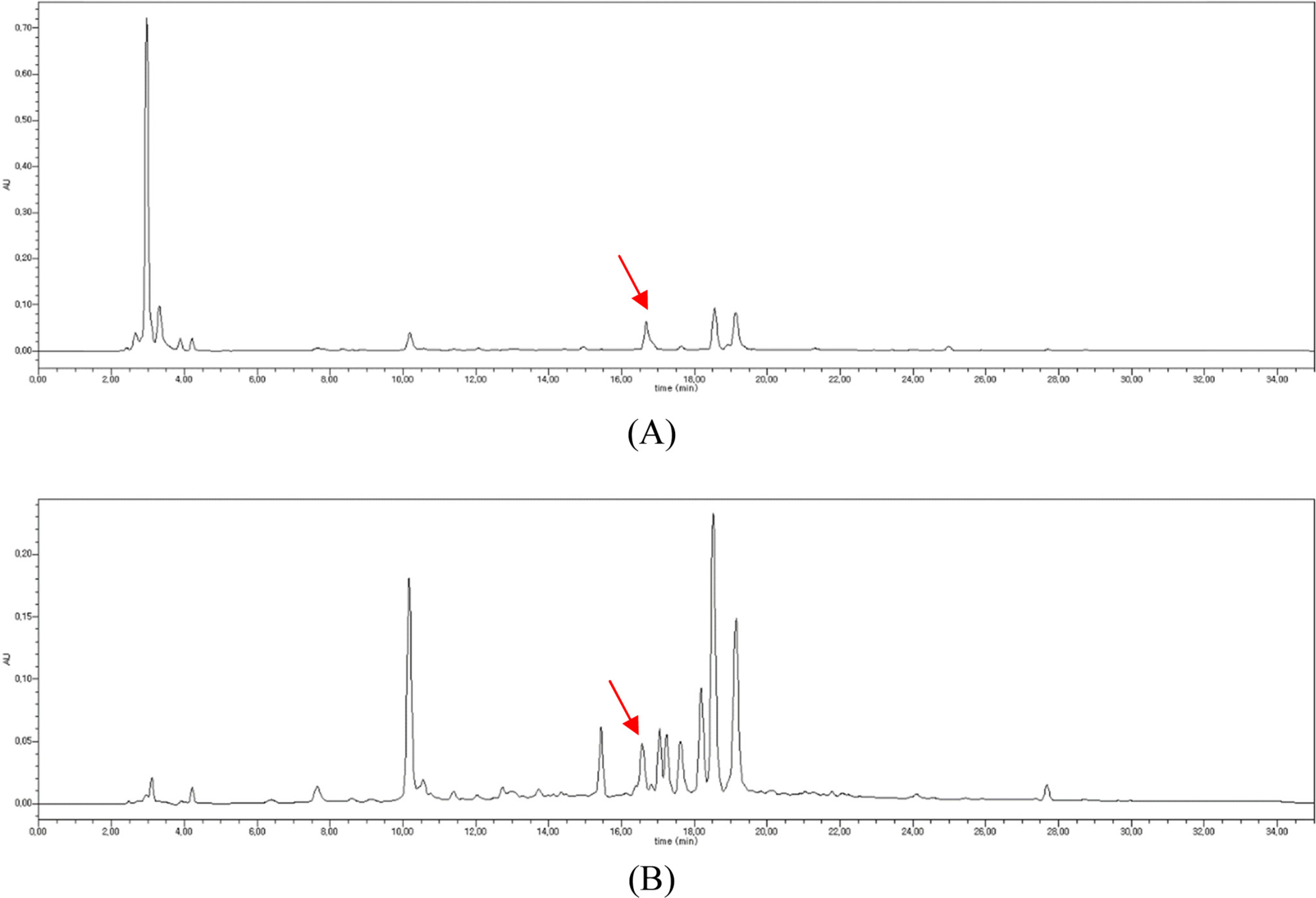
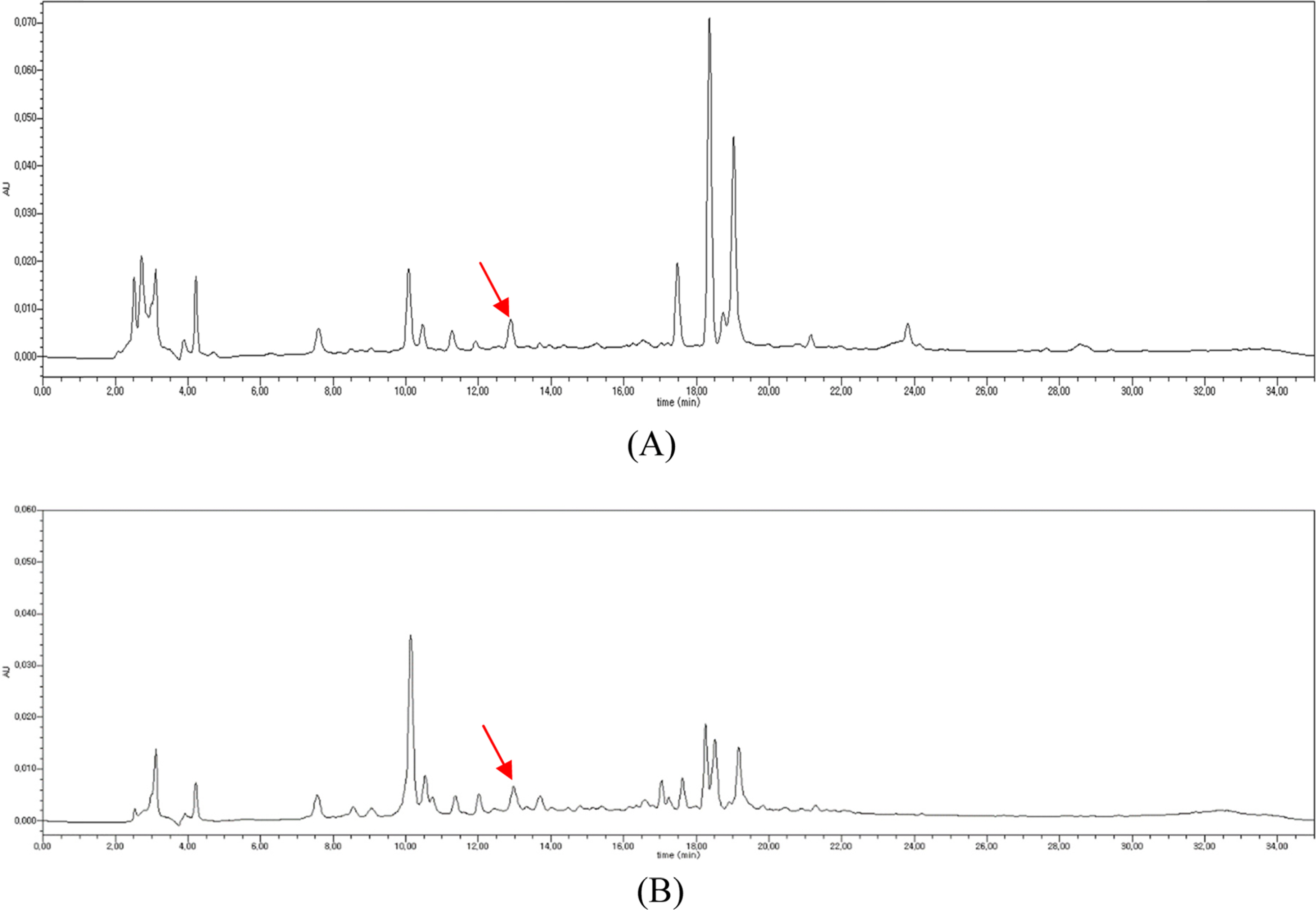
- To determine the optimum extraction conditions that give the highest yield of isoquercitrin and caffeic acid from Aster scaber, the effects of four extraction variables (solvent concentrations, extraction time, number of repeated extraction, and solvent volumes) on isoquercitrin and caffeic acid yield was examined via HPLC-UV. Our results showed that the highest extract and isoquercitrin yield were observed when A. scaber was extracted with 450 mL distilled water for 8 hr repeatedly for three times. In case of caffeic acid, the content was higher in the two repeated extracts. Also, content analysis of isoquercitrin in Aster species was performed in which A. fastigiatus, A. ageratoides, and A. scaber exhibited the highest isoquercitrin content at 6.39, 5.68, and 2.79 mg/g extract, respectively. In case of caffeic acid, the highest content of A. scaber and A. glehni was 0.64 and 0.56 mg/g extract, respectively. This study reports an optimized method for extraction of isoquercitrin and caffeic acid from A. scaber and evaluates potential sources of the compounds.
Keyword
Figure
Reference
-
(1). Jung C. M., Kwon H. C., Seo J. J., Ohizumi Y., Matsunaga K., Saito S., Lee K. R.Chem. Pharm. Bull. 2001; 49:912–914.(2). Kim H. M., Lee D. G., Cho E. J., Choi K., Ku J. J., Park K. W., Lee S. H.Hort. Environ. Biotechnol. 2013; 54:183–189.(3). Nugroho A., Kim K. H., Lee K. R., Alam M. B., Choi J. S., Kim W. B., Park H. J.Arch. Pharm. Res. 2009; 32:1361–1367.(4). Chung T. Y., Eiserich J. P., Shibamoto T. J.Agric. Food Chem. 1993; 41:1693–1697.(5). Kwon H. C., Jung C. M., Shin C. G., Lee J. K., Choi S. U., Kim S. Y., Lee K. R.Chem. Pharm. Bull. 2000; 48:1796–1798.(6). Woo J. H., Jeong H. S., Yu J. S., Chang Y. D., Lee C. H.Korean J. Plant Res. 2008; 21:52–59.(7). Chung M. J., Lee S., Park Y. I., Lee J., Kwon K. H.Life Sci. 2016; 148:173–182.(8). Choi N. S., Oh S. S., Lee J. M.Koeran J. Food Sci. Technol. 2001; 6:745–752.(9). Nugroho A., Kim K. H., Lee K. R., Alam M. B., Choi J. S., Kim W. B., Park H. J.Arch. Pharm. Res. 2009; 10:1361–1367.(10). Kim S. A., Kim J. S.Korean J. Food Sci. Technol. 2012; 6:686–691.(11). Beom S. W., Jiang G. H., Eun J. B.Korean J. Food Preserv. 2015; 22:51–55.
Article(12). Jeong Y. S., Lee S. H., Song J., Hwang K. A., Noh G. M., Jang D. E., Hwang I. G.Korean J. Food Nutr. 2016; 29:767–776.(13). Price K. R., Casuscelli F., Colquhoun I. J., Rhodes M. J. C. J.Sci. Food Agric. 1998; 77:468–472.(14). Rogerio A. P., Kanashiro A., Fontanari C., da Silva E. V., Lucisano-Valim Y. M., Soares E. G., Faccioli L. H.Inflamm. Res. 2007; 56:402–408.(15). Jung S. H., Kim B. J., Lee E. H., Osborne N. N.Neurochem. Int. 2010; 57:713–721.(16). Gasparotto Junior A., Gasparotto F. M., Lourenco E. L., Crestani S., Stefanello M. E., Salvador M. J., de Silva-Santos J. E., Marques M. C., Kassuya A. L. J.Ethnopharmacol. 2011; 134:363–372.(17). Makino T., Kanemaru M., Okuyama S., Shimizu R., Tanaka H., Mizukami H. J.Nat. Med. 2013; 67:881–886.(18). Valentová K., Vrba J., Bancí ová M., Ulrichová J., K en V.Food Chem. Toxicol. 2014; 68:267–282.(19). Clifford M. N. J.Sci. Food Agric. 1999; 79:362–372.
Article(20). Chiou S. Y., Sung J. M., Huang P. W., Lin S. D. J.Med. Food. 2017; 20:140–151.(21). Kim A. R., Jin Q., Jin H. G., Ko H. J., Woo E. R.Arch. Pharm. Res. 2014; 37:845–851.(22). Yun J. E., Woo E. R., Lee D. G. J.Funct. Foods. 2016; 22:347–357.(23). Kim H. K., Kwon Y. J., Kim Y. E., Nahmgung B.Korean J. Food Preserv. 2004; 1:88–93.(24). Thiruvengadam M., Praveen N., Yu B. R., Kim S. H., Chung I. M.Acta Biol. Hung. 2014; 65:144–155.(25). Nugroho A., Kim M. H., Choi J., Choi J., Jung W. T., Lee K. T., Park H. J.Arch. Pharm. Res. 2012; 35:423–430.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Microwave Assisted Extraction, Optimization using Central Composite Design, Quantitative Estimation of Arjunic Acid and Arjunolic Acid using HPTLC and Evaluation of Radical Scavenging Potential of Stem Bark of Terminalia arjuna
- Quantitative Analysis of Bioactive Compounds in a Mixture of the Lindera glauca Leaves Extract and Water-soluble Mastic Gum using an HPLC/UV Method
- Optimization of Cultivation and Extraction Conditions of Pupae-Cordyceps for Cordycepin Production
- Optimization of Extraction Condition of Hesperidin in Citrus unshiu Peels using Response Surface Methodology
- Polyphenols in peanut shells and their antioxidant activity: optimal extraction conditions and the evaluation of antiobesity effects